| disease | Chronic Pericarditis |
| alias | Chronic Pericarditis |
After acute pericarditis, scarring, adhesions, and calcium deposits may remain on the pericardium. Most patients only develop mild scarring and loose or localized adhesions, with no significant thickening of the pericardium and no impact on cardiac function. This condition is termed chronic adhesive pericarditis (chronic adhesive pericarditis) and holds no clinical significance. In some patients, pericardial effusion persists long-term, leading to chronic effusive pericarditis (chronic effusive pericarditis), which may represent the chronic phase of acute nonspecific pericarditis. The primary manifestation is pericardial effusion, and the prognosis is generally favorable. A minority of patients develop dense scar tissue, causing the pericardium to lose elasticity and significantly impairing cardiac systolic and diastolic function. This condition is known as constrictive pericarditis, which includes the classic chronic constrictive pericarditis (chronic constrictive pericarditis) and subacute effusive-constrictive pericarditis (subacute effusive constrictive pericarditis), where pericardial constriction occurs alongside effusion. The latter presents with both pericardial tamponade and constrictive features clinically and eventually progresses to typical chronic constrictive pericarditis. This section primarily discusses the clinical aspects of chronic constrictive pericarditis.
bubble_chart Etiology
Constrictive pericarditis is secondary to acute pericarditis. Sometimes, the clinical transition from acute to constrictive pericarditis can be observed, but in most cases, the symptoms of the acute phase are not obvious. By the time the manifestations of constrictive pericarditis become apparent, the pathological features of the original disease are often lost, making it difficult to determine the {|###|}disease cause{|###|} in many patients. Among the confirmed {|###|}disease causes{|###|}, tuberculous pericarditis accounts for the majority, followed by nonspecific pericarditis. Cases caused by radiation therapy and open-heart surgery are gradually increasing, while a few are due to purulent pericarditis and traumatic pericarditis. Rheumatic pericarditis rarely leads to pericardial constriction. Occasionally, constrictive pericarditis has been reported in cases of rheumatoid arthritis, systemic lupus erythematosus, uremia, histoplasmosis, tularemia, actinomycosis, coxsackievirus B infection, epidemic {|###|}common cold{|###|}, infectious mononucleosis, herpes simplex, salmonellosis, echinococcosis, {|###|}schistosomiasis{|###|}, amebiasis, malignant tumors, pericardial foreign bodies, chylous pericarditis, cholesterol pericarditis, dialysis therapy, renal transplantation, and pericardial hemorrhage following anticoagulant therapy.
Due to advancements in diagnostic techniques, it is now possible to identify cases of constrictive pericarditis accompanied by pericardial effusion, forming subacute effusive-constrictive pericarditis, caused by certain inflammations (viral, associated with primary mediastinal fibrosis, or sarcoidosis), uremia, neoplasms, and trauma (including post-cardiac surgery and radiation therapy).bubble_chart Pathological Changes
In chronic constrictive pericarditis, the visceral and parietal layers of the pericardium are extensively adhered, thickened, and calcified. The pericardial cavity is obliterated, forming a fibrous scar tissue shell that tightly encases and compresses the entire heart and the roots of the great vessels. It can also be localized to certain areas of the heart's surface, such as forming annular constrictions in the atrioventricular groove or the root of the main stirred pulse. On the ventricular surface, especially the right ventricle, the scar tissue is often more rigid and thick, typically ranging from 0.2 to 2 cm or even thicker. In most patients, the scar tissue primarily consists of dense collagen fibers, exhibiting patchy or sheet-like hyaline degeneration, making it impossible to identify characteristic changes indicative of the primary disease. In some patients, subcutaneous nodular or suppurative granulation tissue may still be found within the pericardium.
The frequent discovery of areas encapsulated by a fibrous layer and containing concentrated blood components and bodily fluids suggests that intrapericardial hemorrhage is a significant factor in the formation of pericardial constriction.
The heart's shape may be normal or smaller, and pericardial lesions often affect the underlying myocardium. The constrictive pericardium impairs the heart's activity and metabolism, sometimes leading to myocardial atrophy, fibrous degeneration, fatty infiltration, and calcification.
bubble_chart Clinical ManifestationsThe onset of constrictive pericarditis is often insidious. The manifestations of pericardial constriction appear from several months to several decades after acute pericarditis, typically within 2 to 4 years. In the early stages of constriction development, signs are often more prominent than symptoms, and even in the late stage [third stage], patients with significant circulatory insufficiency may only exhibit mild symptoms.
(1) Symptoms: Exertional dyspnea is often the earliest symptom of constrictive pericarditis, resulting from a relatively fixed cardiac output that cannot adequately increase during activity. In the late stage [third stage], due to massive pleural effusion, ascites elevating the diaphragm, and pulmonary congestion, dyspnea may occur even at rest, and orthopnea may develop. Massive ascites and an enlarged liver compress the abdominal organs, causing a sensation of abdominal distension. Additionally, symptoms may include lack of strength, loss of appetite, vertigo, weakness, palpitation, cough, epigastric pain, edema, etc.
(2) Signs
1. Cardiac manifestations: The cardiac dullness border may be normal or slightly enlarged. The apical impulse is weakened or absent, and the heart sounds are faint and distant, which are related to restricted cardiac activity and reduced cardiac output. The pulmonary component of the second heart sound may be accentuated. Some patients may hear an early diastolic extra sound (pericardial knock) approximately 0.1 seconds after the second heart sound in the third to fourth intercostal spaces along the left sternal border, similar to that observed in acute pericarditis with cardiac tamponade. The heart rate is often rapid. The rhythm is generally sinus, but ectopic rhythms such as premature beats, atrial fibrillation, or atrial flutter may occur.
2. Manifestations of cardiac compression: Jugular vein distension, hepatomegaly, ascites, pleural effusion, lower limb edema, etc. These are related to impaired cardiac diastolic function, which reduces cardiac output, leading to renal retention of water and sodium, thereby increasing blood volume, and elevated venous pressure due to obstructed venous return. In constrictive pericarditis, ascites appears earlier and is often more severe than subcutaneous edema, differing from general heart failure. The exact reason remains unclear but may be associated with the following factors: ① A slow and progressive increase in venous pressure causes spasm of subcutaneous small arteries but not visceral small arteries; ② Pericardial adhesions are most prominent near the hepatic veins entering the inferior vena cava, leading to severe hepatic venous congestion and significant obstruction of abdominal lymphatic drainage, making it easier for edema fluid to accumulate in the abdominal cavity; ③ Reduced renal blood flow is less pronounced, and water and sodium retention is mild, resulting in delayed and milder subcutaneous edema, primarily distributed in the lower limbs and lumbosacral region. Additionally, pleural effusion may develop at some point during the course of the disease. Pulsus paradoxus may sometimes occur. Reduced cardiac output lowers arterial systolic pressure, while venous congestion reflexively causes peripheral small artery spasm, raising diastolic pressure, thus narrowing the pulse pressure.bubble_chart Auxiliary Examination
Laboratory Tests There are no characteristic changes, but grade I anemia may be present. In patients with a prolonged course, liver congestion often leads to impaired liver function, resulting in reduced plasma protein production, especially albumin. Some patients may have persistent proteinuria due to renal congestion, exacerbating hypoalbuminemia. Ascites and pleural effusions are typically transudative in nature. Venous pressure is significantly elevated and further rises during inspiration (Kussmaul's sign). Circulation time is prolonged.
Electrocardiogram (ECG) Findings Low QRS voltage and flat or inverted T waves are observed. The simultaneous presence of both strongly supports the diagnosis of constrictive pericarditis. T-wave changes without low voltage are clinically helpful, whereas low voltage without T-wave changes is nonsignificant. ECG changes often indicate the extent and severity of myocardial involvement. About 50% of cases show broad, notched P waves. Right ventricular hypertrophy or right bundle branch block may occur, and atrial fibrillation is not uncommon, particularly in older patients with long-standing disease.
X-ray Examination Pericardial calcification is the most reliable radiographic sign of prior acute pericarditis and is seen in most cases of constrictive pericarditis, often appearing as an incomplete ring. Over half of patients exhibit grade I cardiomegaly, while the rest have a normal-sized heart. Cardiac enlargement may be related to pericardial thickening, residual pericardial effusion, diaphragmatic elevation, or thickening of adjacent pleural membranes. The heart may appear diffusely enlarged, triangular, or globular, with straightened borders or abnormal contours—such as shortening or obscuration of the aortic knob, enlargement of the left or right atrium, right ventricle, or pulmonary conus, and dilation of the superior vena cava. Increased lung markings, pulmonary vascular congestion, and pleural thickening or effusion may also be present. Fluoroscopy or kymography may reveal weakened or absent cardiac pulsations. Cardiac angiography can demonstrate chamber sizes and dynamic morphological changes during the cardiac cycle, aiding in estimating pericardial thickness and the degree of constriction.
Computed Tomography (CT) CT has high specificity and resolution for detecting pericardial thickening. If the parietal pericardium is thickened by 0.5–2 cm, the imaging curve will show dense tissue, suggesting thickening (Figure 1). Magnetic Resonance Imaging (MRI) can differentiate pericardial thickening and assess the presence of constriction.
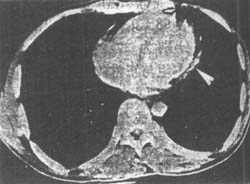
Figure 1 Contrast-enhanced CT shows pericardial thickening (arrow) posterior to the left ventricle, measuring 6.7 mm.
Cardiac Catheterization The key hemodynamic features of constrictive pericarditis on right heart catheterization include markedly elevated and nearly equal pressures in the pulmonary capillary wedge, pulmonary artery diastolic, right ventricular end-diastolic, right atrial mean, and central venous pressures, along with reduced cardiac output. The right atrial pressure curve exhibits an M-shaped pattern, with nearly equal a and v waves. The right ventricular pressure curve shows an early diastolic dip and a late diastolic plateau. Additionally, the right atrial pressure curve rises during breath-holding after inspiration.
If the patient exhibits signs of systemic venous congestion such as ascites, hepatomegaly, distended neck veins (more pronounced during inspiration, with diastolic collapse), and significantly elevated venous pressure, without significant cardiac enlargement or heart valve murmurs, constrictive pericarditis should be considered. If there is also a history of acute pericarditis, weakened cardiac impulse, an early diastolic extra sound, narrowed pulse pressure, paradoxical pulse, and lower limb edema, along with X-ray findings of pericardial calcification and ECG changes in the QRS complex, T wave, and P wave, the diagnosis can often be confirmed. Further evaluation with computed tomography (CT) or magnetic resonance imaging (MRI) may be performed to assess pericardial thickening. In atypical cases, right heart catheterization may be required.
bubble_chart Treatment Measures
Pericardial stripping should be performed as early as possible. If the disease course is prolonged, myocardial atrophy and fibrosis often occur, which can affect the surgical outcome. Therefore, as long as the clinical manifestations indicate progressive cardiac compression that cannot be explained by simple pericardial effusion, or if signs of cardiac compression become increasingly evident during the absorption of pericardial effusion, or if significant thickening of the parietal pericardium is observed during pericardial air insufflation, or if magnetic resonance imaging reveals pericardial thickening and constriction—provided the pericardial infection is largely under control—surgery should be performed as early as possible. Patients with subcutaneous node pericarditis should undergo surgery only after the subcutaneous node activity has subsided to avoid premature surgery leading to the spread of subcutaneous node. If the subcutaneous node is not yet stable but cardiac compression symptoms worsen significantly, surgery may be performed under aggressive anti-subcutaneous node therapy. During the operation, the pericardium should be stripped as thoroughly as possible, especially the pericardium covering both ventricles. Due to long-term cardiac constriction, myocardial atrophy and fibrosis are common, so the cardiac workload should not be increased immediately after surgery; activity should be gradually increased. Intravenous fluid replacement must be administered cautiously to avoid acute pulmonary edema. Since the recovery of atrophied myocardium is slow, successful surgical outcomes often become apparent only 4–6 months postoperatively.
Before surgery, the patient's general condition should be improved through strict rest, a low-salt diet, diuretics, or drainage of pleural effusion and ascites. If necessary, small, frequent blood transfusions may be given. Patients with heart failure or atrial fibrillation may be appropriately treated with Rehmannia-based medications.
If a thorough pericardiectomy is performed early, most patients can achieve satisfactory results. However, a small number of patients with a prolonged course of the disease, marked myocardial atrophy, and severe conditions such as cardiac cirrhosis have a poorer prognosis.
The clinical manifestations of constrictive pericarditis and restrictive primary cardiomyopathy are extremely similar, making differentiation often very difficult. Table 1 can serve as a reference for diagnosis.
Table 1: Differentiation between Constrictive Pericarditis and Restrictive Cardiomyopathy
| Differentiation Item | Constrictive Pericarditis | Restrictive Cardiomyopathy | |
| Fatigue and Dyspnea | Gradual onset, later becoming obvious | Obvious from the start | |
| Jugular Venous Distension during Inspiration | Present | Absent | |
| Apical Impulse | Often not palpable | Often palpable | |
| Pulsus Paradoxus | Often present | Absent | |
| Murmurs of Mitral and Tricuspid Regurgitation | Absent | Often present | |
| Diastolic Heart Sounds | Occur early after the second heart sound, louder, as an early diastolic extra sound (pericardial knock) | Occur later after the second heart sound, softer, as a third heart sound, often with audible fourth or sixth sounds | |
| X-ray | Grade I cardiac enlargement, common pericardial calcification | Often significant cardiac enlargement, no pericardial calcification, possible endocardial membrane calcification | |
| Electrocardiogram | Low QRS voltage and generalized T-wave changes, may have atrial fibrillation or P-wave changes suggesting left atrial hypertrophy | May have low QRS voltage and generalized T-wave changes, sometimes abnormal Q waves, often atrioventricular and intraventricular conduction blocks (especially left bundle branch block) and ventricular hypertrophy with strain, may also have atrial fibrillation | |
| Systolic Time Interval Measurement | Normal | Abnormal (PEP prolonged, LVET shortened, PEP/LVET ratio increased) | |
| Echocardiogram | Significant atrial enlargement | Uncommon | Common |
| Early Diastolic Mitral Inflow Velocity | Marked respiratory variation | Minimal respiratory variation | |
| Ventricular Filling Discordance | Present | Absent | |
| Hemodynamic examination | Left and right ventricular end-diastolic pressure | Equal (difference ≤ 0.67 kPa (5 mmHg)) | >0.67 kPa (5 mmHg) |
| Right ventricular systolic pressure | ≤50 mmHg | >50 mmHg | |
| Right ventricular end-diastolic pressure | >1/3 right ventricular systolic pressure | <1/3 right ventricular systolic pressure | |
| Computed tomography (CT) imaging | Pericardial thickening | Normal pericardium | |
| Endomyocardial biopsy | Normal | Abnormal | |
| Rehmannia treatment response | Venous pressure unchanged | Venous pressure decreased | |
Since surgical treatment for constrictive pericarditis often yields favorable outcomes, while cardiomyopathy has a poor prognosis, cases with significant diagnostic difficulty should undergo hemodynamic and imaging (CT or MRI) studies, and endomyocardial biopsy if necessary. If imaging shows pericardial thickening, thoracotomy should be considered unless all three hemodynamic tests meet the criteria for restrictive cardiomyopathy. If endomyocardial biopsy reveals myocardial disease, thoracotomy is unnecessary. Additionally, differentiation is required from cirrhosis, subcutaneous nodular peritonitis, and heart failure caused by other cardiac conditions.





