| disease | Malignant Tumor of the Maxillary Sinus |
According to domestic data, statistical analysis of tumors in the otolaryngology department shows that malignant tumors of the maxillary sinus account for 40.3% of nasal malignant tumors and 1.2% of systemic malignant tumors. Lewis et al. (1972) analyzed 772 cases of nasal and paranasal sinus cancer, with approximately 30% occurring in the nasal cavity and 70% in the paranasal sinuses, with the majority occurring in the maxillary sinus, accounting for 58%. This disease is more common in individuals over 50 years old, with a male-to-female incidence ratio of 2:1.
bubble_chart Pathological Changes
According to domestic and international literature reports, malignant tumors of the maxillary sinus are primarily squamous cell carcinoma, accounting for approximately 80%; followed by undifferentiated carcinoma, adenocarcinoma, mucoepidermoid carcinoma, cylindric cell carcinoma, lymphoepithelial carcinoma, papillary carcinoma, malignant melanoma, malignant plasmacytoma, and chondrosarcoma or osteosarcoma.
bubble_chart Clinical Manifestations
Malignant tumors of the maxillary sinus, due to their large cavity and varying primary sites, may be asymptomatic in the early stages and are often discovered during examinations. As the tumor gradually grows and affects surrounding tissue structures and functions, corresponding symptoms and signs may arise. If the tumor develops towards the nasal cavity, symptoms may include a stuffy nose, bloody and foul-smelling mucopurulent nasal discharge. Nasal examination may reveal medial displacement of the lateral nasal wall leading to nasal stenosis. Sometimes, masses may be found in the middle nasal meatus or nasal cavity, which is an opportune time for biopsy to confirm pathological diagnosis. If the tumor invades the nasolacrimal duct, epiphora may occur. Involvement of the anterior wall of the maxillary sinus can cause cheek swelling, deformity, and facial numbness and pain. If the tumor infiltrates the floor, patients often experience toothache, gum swelling, loose or lost teeth, and a semicircular bulge of the hard palate, which can be misdiagnosed as dental disease. Symptoms may worsen after tooth extraction. The tumor can also develop towards the posterior wall of the maxillary sinus, invading the pterygopalatine fossa and causing difficulty in opening the mouth. If the tumor destroys the orbital floor or enters the orbit, it can lead to eyeball displacement and visual impairment. In advanced stages, the tumor can invade the anterior cranial fossa through the ethmoid sinus and orbit, or through the pterygomaxillary and pterygopalatine fossae, destroying the roof of the pterygopalatine fossa or involving the infratemporal fossa and entering the middle cranial fossa. Clinical symptoms such as a mass in the inner canthus, difficulty in opening the mouth, neck swelling, persistent headache, and ear pain may indicate possible skull base or intracranial metastasis. About half of malignant tumors of the maxillary sinus have lymph node metastasis.
bubble_chart DiagnosisMalignant tumors of the maxillary sinus are difficult to diagnose early due to the absence of symptoms and signs. By the advanced stage, when various symptoms become apparent, diagnosis is usually not difficult. In recent years, with the gradual popularization of high-resolution CT and MRI imaging examinations and the clinical application of multi-angle sinus endoscopy, early detection of sinus tumors has become possible. Any mass found in the middle nasal meatus and imaging suggesting a space-occupying lesion in the sinus should prompt an early biopsy for pathological examination. To correctly understand the biological characteristics of malignant tumors of the maxillary sinus for the purpose of localization diagnosis, selection of surgical methods, and prognosis estimation, several localization methods and classifications of maxillary sinus cancer are introduced below.
1. Ohngren's method: A hypothetical oblique plane is drawn between the inner canthus and the mandibular angle, and a hypothetical vertical plane is drawn at the pupil, dividing the maxillary sinus into four quadrants. Tumors growing in the anteromedial quadrant are prone to invade the ethmoid sinus, causing nasal symptoms and swelling in the inner canthus. Tumors in the posterolateral quadrant in the advanced stage are prone to destroy the posterior wall, invade the pterygomaxillary and pterygopalatine fossae, and may further destroy the roof of the pterygopalatine fossa or enter the infratemporal fossa, involving the middle cranial fossa, leading to symptoms such as difficulty in opening the mouth, temporal swelling, headache, and ear pain. Tumors located in the lower part may first present with dental symptoms, such as gum swelling, loose teeth, and tooth loss.
2. Sebileau's method: A hypothetical horizontal plane is drawn from the lower edge of the middle nasal turbinate, dividing the maxillary sinus into upper and lower parts. Tumors in the upper part are prone to invade the ethmoid sinus or orbit, causing nasal and ocular symptoms, and may further invade the skull base. Tumors occurring in the lower part have a better prognosis than those in the upper part.3. Lederman's method: Two horizontal lines are drawn from the orbital floor and the floor of the maxillary sinus, and two vertical lines are drawn from the medial walls of both orbits through the nasal floor, dividing the maxillary region into upper, middle, and lower parts. The vertical lines serve as the boundaries between the ethmoid sinus, nasal cavity, and the upper maxillary sinus. The nasal septum naturally separates the ethmoid sinuses and nasal cavities on both sides. The main advantage of this classification is that it essentially encompasses the entire maxillary region. The anatomical structures included in the upper, middle, and lower parts are as follows:
(1) Upper region: Ethmoid sinus, frontal sinus, sphenoid sinus (not involving the nasopharynx), olfactory region of the nasal cavity (i.e., the part above the middle turbinate).
(2) Middle region: Laterally, the maxillary sinus; medially, the respiratory part of the nasal sinus, nasal vestibule, and lateral wall of the nasal cavity (including the inferior turbinate).
(3) Lower region: Floor of the maxillary sinus, nasal vestibule, tumors simultaneously invading the maxillary sinus and hard palate, floor of the nasal cavity and hard palate, odontogenic tumors.
The TNM staging of malignant tumors of the maxillary sinus is as follows:
Stage I: T1N0M0;Stage II: T2N0M0;
Stage III: T1, 2N1M0; T3N0M0 with lymph node metastasis or suspected lymph node metastasis.
Stage IV: T4N0M0, T4N1M0, T1, 2, 3N2M0, 1. Fixed lymph node metastasis or distant organ metastasis. Sakai et al. (1976) staged maxillary sinus cancer as follows:
Stage I: T1~2N0M0.
Stage II: T3N0M0.
Stage III: T4N0M0; T1~4N1M0.
Stage IV: T1~4N2~3M0; T1~4N0~2M1.
The localization and classification of malignant tumors of the maxillary sinus are standards developed by doctors after dynamic observation of the whole body and local areas of the disease, for diagnosis, treatment, and prognosis judgment, which can be used to standardize doctors' diagnostic and therapeutic behaviors.
bubble_chart Treatment Measures
Malignant tumors of the maxillary sinus are currently treated primarily with surgical resection, supplemented by radiotherapy or chemotherapy as part of a comprehensive treatment approach.
(1) Radiotherapy is often recommended in conjunction with surgical resection. However, there is no complete consensus on whether it should be applied preoperatively or postoperatively. Preoperative radiotherapy in appropriate doses can reduce tumor size and decrease lymphatic metastasis, as radiation therapy can lead to insufficient blood supply to the tumor and low oxygen tension in the tissues, thereby reducing the tumor's sensitivity to radiation. Postoperative radiotherapy serves as a supplementary treatment for residual active cells at the surgical margins and for lymphatic vessels and nodes that are difficult to reach and have already metastasized. For patients with advanced-stage tumor diseases who have lost the opportunity for surgery, radiotherapy can prolong their lives.
(2) Surgical therapy: The choice of surgical procedure should be based on the primary site of the lesion and the extent of invasion.
1. Partial maxillectomy: Suitable for malignant tumors of the maxillary sinus limited to the floor of the maxillary sinus or early malignant tumors of the alveolar process and hard palate.
A long incision is made in the gingiva on the affected side, cutting through the mucous membrane and periosteum. The soft tissue of the hard palate is incised medially to the central incisor on the affected side, extending to the junction of the hard and soft palate, then laterally to the third molar, connecting with the incision on the anterior wall of the maxillary sinus. Within the scope of the above incision, the hard palate including the dental portion of the maxilla is removed with a bone chisel or saw, fully exposing the maxillary sinus and nasal cavity. Finally, the nasal wall of the maxillary sinus is removed to connect the maxillary sinus cavity with the nasal cavity. The wound cavity after tumor removal is packed with iodoform gauze, which is removed 4-6 days postoperatively, and a pre-prepared denture base is installed to close the wound, restore the patient's chewing and speech functions, and prevent deformity due to soft tissue contraction.
2. Maxillectomy: Maxillectomy is a common surgery performed by otolaryngologists for malignant tumors primarily involving the maxillary sinus. In recent years, with the development of surgical techniques, the basic surgical approach has undergone significant modifications.
Maxillectomy usually employs the Weber-Fergusson incision. Here, the modified Dieffenbach-Weber-Fergusson incision is mainly introduced (Figures 1, 2). This incision has the following advantages: ① Easy access to the soft tissues of the cheek and elevation of the cheek flap under direct vision; ② Direct observation of the cheek flap; ③ Direct observation of whether the cheek soft tissues and flap are infiltrated by the tumor and the extent of infiltration; ④ The nasal side incision can be extended upward to the eyebrow arch, and the infraorbital rim incision can be extended laterally to the temporal region as needed; ⑤ A "W" shaped incision made on the nasolabial fold can prevent upward and forward displacement deformity of the upper lip due to incision contraction and avoid the formation of obvious linear scars.
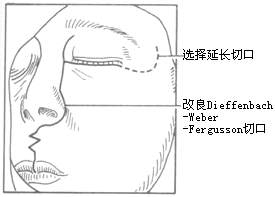
Figure 1: Incision for maxillectomy
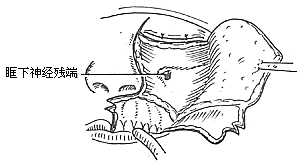
Figure 2: Elevation of the flap to both sides
To ensure an accurate and neat incision, the skin should be marked with Chinese Gentian violet before surgery. To reduce bleeding, infiltrate anesthesia with 0.5-1% lidocaine (with an appropriate amount of 1:1000 epinephrine) along the incision and cheek area. Ligate the stirred pulse at the incision site. The incision includes the nasal margin, upper lip, labial-gingival groove, inner canthus, lower eyelid, etc. The incision at the inner canthus should avoid injuring the inner canthus ligament. The lower eyelid incision should be made 2-3 cm from the eyelid margin in an arc-shaped horizontal cut extending below the outer canthus, cutting through the skin and subcutaneous tissue, and aligning with the infraorbital rim, reaching the bone membrane. The incision should align as much as possible with the direction of the orbicularis oculi muscle fibers. When separating the infraorbital rim bone membrane, care should be taken to avoid penetrating the bone membrane and entering the orbit. The infraorbital nerve and blood vessels should be ligated and cut. If the maxilla with the preserved infraorbital wall is to be removed, the lower eyelid incision can be changed to an intra-conjunctival sac incision, parallel to the lower eyelid margin, from the inner canthus to the outer canthus, sutured with fine silk thread postoperatively, leaving no scar on the lower eyelid, which has a cosmetic effect. Place a retractor through the infraorbital bone membrane to gently lift the eyeball, locate the nasolacrimal duct and transect it. Depending on the extent of tumor involvement, lift or remove the remaining part of the lacrimal duct from the lacrimal sac fossa. Separate backward along the lamina papyracea, using bipolar coagulation to block the anterior and posterior stirred pulse (Figures 3, 4). After completing the above soft tissue incisions, flip the cheek flap outward and protect it with saline gauze. At this point, the anterior part of the maxilla, the posterolateral part, the infraorbital rim, the frontal process of the maxilla, the alveolar process, the zygomatic bone, the pear-shaped aperture rim, and the lateral wall of the nasal cavity are all exposed within the surgical field. Use a dissector to separate the pear-shaped aperture and the lateral wall mucous membrane of the nasal cavity, fully separating the bone wall from the mucous membrane. From between the two central incisors, cut along the midline of the hard palate backward through the mucoperiosteum of the hard palate to the posterior edge of the hard palate, then turn the blade outward and perpendicular to the upper incision, cut along the posterior edge of the hard palate, fully incising the junction of the soft and hard palate on the same side, reaching the posterior edge of the second molar, meeting the end of the labial-gingival groove incision, separate the incised mucoperiosteal flap to both sides, simultaneously extract the affected central incisor, split the hard palate bone from the midline, cut the lateral wall of the nasal cavity, after fully separating the infraorbital bone membrane, first locate the anterior end of the infraorbital fissure, then free the attached soft tissue from the zygomatic process of the maxilla and the lower edge of the zygomatic bone, insert a wire saw or bone scissors from the anterior end of the infraorbital fissure, cut the zygomatic bone. Use a finger to locate the maxillary tuberosity behind the third molar, use a large flat chisel or bone scissors to cut the connection between the maxillary tuberosity and the pterygoid process of the sphenoid bone (Figures 5, 6). At this point, the entire maxilla is basically loosened, use bone-holding forceps to grasp the body of the maxilla and shake it in all directions, if there are still parts not cut, use scissors to supplement the cut, remove the maxilla along with the tumor. If there is bleeding from the intra-maxillary stirred pulse, it should be ligated. Fill the wound cavity with hot saline gauze and compress for 5 minutes, then remove and check the wound cavity for bleeding, residual tumor, and whether the safety margin is sufficient. The wound cavity can be filled with antibiotic-laden Vaseline gauze or iodoform gauze, the incision is sutured in two layers, with pressure dressing applied (Figure 7).
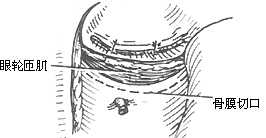
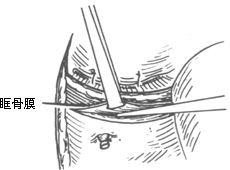
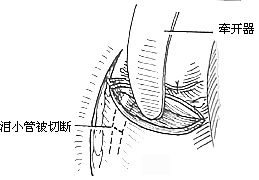
Figure 3 Separation of the upper wall of the maxilla
(1) Infraorbital rim incision (2) Freeing the orbital floor membrane (3) Transection of the nasolacrimal duct
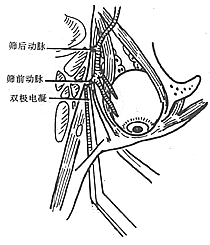
Figure 4 Electrocoagulation of the anterior and posterior ethmoid stirred pulse
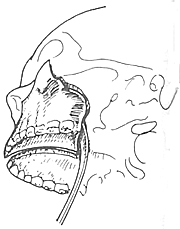
Figure 5 Chiseling the connection between the maxillary tuberosity and the pterygoid process
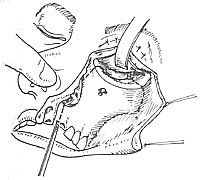
Figure 6 Midline splitting of the hard palate
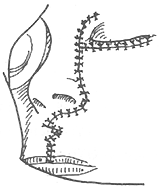
Figure 7 Suture of the incision
Some issues regarding maxillectomy remain controversial: ① Regarding the issue of skin grafting in the wound cavity: Proponents of skin grafting believe that it can accelerate epithelialization of the wound surface, avoiding long-term exudation, scabbing, and granulation tissue proliferation. However, epithelialization can also occur naturally without skin grafting, albeit over a longer period, hence most scholars believe the benefits of skin grafting outweigh the drawbacks. ② Regarding the management of hard palate defects: If only the hard palate bone is involved, the hard palate mucoperiosteum can be preserved after resection of the hard palate bone. If the hard palate bone and mucoperiosteum are both involved, the defect can be repaired by nasal septum displacement after total resection. The traditional method involves installing a dental prosthesis to ensure normal eating and speech communication post-surgery. ③ Repair of orbital floor defects: After resection of the orbital floor bone due to malignant tumors of the maxillary sinus involving the orbital floor, if the membrane capsule is intact, reconstruction may not be necessary, or the nasal septum can be transferred for reconstruction. Partial defects of the orbital floor can be reconstructed using autologous bone or artificial materials to prevent herniation of orbital contents into the wound cavity causing eyeball displacement. ④ Exenteration of orbital contents: Mainly applicable to patients where the tumor has penetrated the orbital membrane capsule or involved the retrobulbar area causing blindness. After total exenteration of the orbital contents, if the conjunctival sac can be preserved and the orbital floor reconstructed, an artificial eye can still be installed; otherwise, a pedicled myocutaneous flap can be used to repair the intraorbital defect. ⑤ Alveolar process resection: It is rare for maxillary sinus cancer to involve the entire alveolar process bone, hence it is currently advocated that patients with tumor infiltration at the base of the maxillary sinus can still undergo maxillectomy with partial preservation of the alveolar process.
3. Radical maxillectomy Radical maxillectomy is suitable for malignant tumors of the maxilla that have extensively invaded the pterygopalatine fossa, pterygomaxillary space, infratemporal fossa, or skull base.
The surgical steps are basically the same as for maxillectomy. However, modifications should be made based on the extent of tumor invasion:
(1) Incision Extend the Dieffenbach-Weber-Fergusson incision outward or upward as appropriate.
(2) Flip the cheek flap outward and downward to expose a larger area of the anterior wall of the maxilla, pear-shaped aperture, lateral wall of the nasal cavity, frontal process of the maxilla, nasal bone, lateral infraorbital rim, buccinator muscle, masseter muscle, temporomandibular joint, anterior segment of the parotid gland, part of the ascending ramus of the mandible, and the lower part of the frontal and temporal muscles.
(3) Expose the ascending ramus of the mandible, cut the attachment of the masseter muscle from the lower edge of the zygomatic arch, and flip the masseter muscle downward. Cut the temporalis muscle from the zygomatic arch, flip the temporalis muscle upward, sever the temporomandibular ligament and the mandibular joint capsule to dislocate the mandible, free the mandibular condyle, cut the lateral pterygoid muscle attached to it, and section the middle segment of the zygomatic arch. This provides a good field of view for further removal of tumors in the infratemporal fossa and pterygopalatine fossa.
(4) Cut the ascending ramus of the mandible and separate the soft tissues attached to the medial region of the ascending ramus. After achieving adequate hemostasis, use a wire saw to cut the ascending ramus below the neck, remove or flip it forward. At this point, the infratemporal fossa, lateral region of the oral cavity, inferior orbital fissure, sphenoid bone, lateral region of the lateral pterygoid plate, and the posterior lateral wall of the maxilla are all well exposed. If the lateral pterygoid muscle is already invaded by the tumor, it can be separated from the medial pterygoid muscle and excised. If the pterygopalatine fossa is involved by tumor growth, it should be removed along with the pterygoid process. However, due to the rich blood supply from the pterygoid plexus, attention should be paid to hemostasis. Ligation of the external carotid and internal maxillary arteries can help reduce intraoperative bleeding and facilitate tumor removal.
(5) Remove the orbital floor. Depending on the extent of orbital floor involvement, a total or partial orbital floor resection can be performed. The postoperative defect management follows the aforementioned methods.
(6) Completely excise the connections between the zygomatic bone, inferior orbital wall, lateral nasal wall, hard palate, maxillary tuberosity, and the pterygoid process of the sphenoid bone, along with the sinus or nasal cavity tumor en bloc. The bone ends can be smoothed with a bone file to reduce postoperative discomfort or headache caused by bone spurs. Radical maxillectomy often leads to facial collapse deformity, and corrective surgery can be performed at the initial stage [first stage] or intermediate stage [second stage].





