| disease | Chronic Constrictive Pericarditis |
Chronic constrictive pericarditis is a chronic inflammatory process involving both the parietal and visceral layers of the pericardium. It leads to pericardial fibrosis and thickening, restricting the diastolic activity of the heart and thereby reducing cardiac function.
bubble_chart Etiology
The main cause of chronic constrictive pericarditis is subcutaneous nodule bacterial infection. Bacteriological and histological examinations confirm subcutaneous nodule lesions in 30% of cases. In approximately 50% or more of cases, the exact causative factor cannot be determined. However, many cases result from prolonged anti-subcutaneous nodule drug therapy, where evidence of subcutaneous nodule lesions has disappeared by the time pericardial constriction occurs. Therefore, it is believed that the majority of these cases are subcutaneous nodule pericarditis, followed by purulent infections. Traumatic and non-traumatic pericardial hemorrhage leading to constrictive pericarditis accounts for about 10%. In recent years, the incidence of this disease following cardiac surgery has increased.
The main pathophysiological change of constrictive pericarditis is the restriction of normal activity in both ventricles due to the constricted pericardium. In the early stage of the disease, it primarily manifests as ventricular diastolic dysfunction. As the condition progresses, the intermediate stage (second stage) of diastole is also significantly affected. During left ventricular diastole, the intraventricular pressure rises rapidly, obstructing blood return in both the left and right ventricles, leading to elevated venous pressure. This presents as jugular vein distension, hepatomegaly, ascites, pleural effusion, and systemic edema. A few patients may also develop splenomegaly. Cardiac output is slightly lower than normal, with a significant reduction in stroke volume. During physical activity or in cases of severe constriction, the body primarily relies on increasing heart rate to maintain minute cardiac output. When annular constriction occurs in the atrioventricular groove or at the roots of the great vessels, corresponding murmurs and signs of valvular dysfunction may appear. A characteristic feature of this disease is the disproportionate degree of ascites compared to peripheral edema. The mechanism of ascites formation involves three main factors: ① passive congestion of the liver due to obstructed hepatic venous return; ② pericardial adhesions at the diaphragmatic surface affecting lymphatic drainage; and ③ decreased plasma albumin levels.
bubble_chart Pathological ChangesThe pericardium is diffusely thickened, but the degree of thickening varies in different regions, with more pronounced thickening observed in the bilateral ventricles and diaphragmatic pericardium. The thickened pericardium is composed of fibrous tissue. Calcium salt deposits may form patchy or band-like calcifications or even a complete bony shell. In the early stages, there may be effusion in the pericardial cavity, and a thin layer of fibrin or fibrous tissue may adhere to the epicardium. As the disease progresses, the layers of the pericardium gradually develop from mild adhesions to tight adhesions and eventually complete fusion, where no distinct boundary remains between the visceral and parietal layers. The thickened pericardium may also adhere to the diaphragm, pleura, and mediastinal structures. In the early stages of constrictive pericarditis, subepicardial myocardial atrophy occurs, while in advanced stages, widespread atrophy leads to a significantly thinner ventricular wall compared to normal. Chronic inflammatory infiltration may also cause focal myocarditis, resulting in partial myocardial fibrosis. In rare cases, annular stenosis may occur in the atrioventricular groove.
Apart from the heart, passive congestion and fibrotic changes may occur in the lungs, liver, spleen, and other organs, similar to the changes caused by long-term heart failure.
bubble_chart Clinical Manifestations
Half of the patients experience a slow onset of symptoms, with an insidious appearance and no history of acute pericarditis. About 30% of patients have a history of acute pericarditis several months prior, with symptoms alleviated after treatment but gradually worsening again. The duration of the disease varies among patients, with some lasting over a decade. Most patients have a history of 1.5 to 2 years by the time major symptoms appear and a definitive diagnosis is made. Common primary symptoms include dyspnea, abdominal distension and fullness, peripheral edema, fatigue, and cough. All patients exhibit varying degrees of dyspnea, with shortness of breath occurring even during mild physical activity; severe cases may present as orthopnea. Dyspnea is primarily caused by pleural effusion or ascites accompanied by elevated diaphragm, leading to reduced lung capacity. Although pulmonary venous pressure increases, pulmonary interstitial edema is rare. Therefore, paroxysmal nocturnal dyspnea and acute pulmonary edema are uncommon. Abdominal distension and fullness result from hepatomegaly, ascites, and visceral congestion. Reduced renal blood flow leads to water and sodium retention, causing peripheral edema, often manifested as ankle edema. Concurrent symptoms may include palpitations, fatigue, loss of appetite, and upper abdominal discomfort. Additionally, cough and precordial dull pain are also relatively common.
Patients exhibit a chronic, sexually transmitted disease-like appearance, with facial edema, engorged superficial veins, and jugular vein distension. Friedrich's sign may be observed, characterized by early diastolic collapse during jugular vein pulsation. When constriction severely affects right ventricular blood return, marked jugular vein distension during inspiration (Kussmaul's sign) may be noted. If pleural effusion is significant, intercostal spaces may widen. Half of the patients exhibit weakened or absent apical impulse, with normal or slightly enlarged cardiac borders on percussion. Sometimes, retraction of the apical and left parasternal regions during systole and rapid outward movement during early diastole may be observed. Heart rate is typically rapid. About two-thirds of patients exhibit an early diastolic third heart sound due to rapid ventricular filling in early diastole. All patients present with abdominal distension, hepatomegaly, and positive ascites signs. Approximately 10% of patients develop splenomegaly. Blood pressure is normal or low, with reduced systolic pressure. Venous pressure is elevated, and both systemic and pulmonary circulation times are prolonged. Patients often exhibit pulsus paradoxus, where the pulse weakens or becomes imperceptible during inspiration. Blood pressure measurements show a drop of more than 10 mmHg in systolic pressure during inspiration compared to expiration. Some attribute these changes to the thickened pericardium adhering to the diaphragm, which, during inspiration, pulls on the pericardium, increasing tension and restricting cardiac filling, thereby causing a sudden reduction in cardiac output and a drop in stirred pulse systolic pressure. bubble_chart Auxiliary ExaminationElectrocardiogram (ECG): All patients exhibited abnormal ECG findings, but none showed specific ECG changes. Most patients had low voltage in ventricular complexes. Approximately 70% of patients displayed P wave abnormalities, including widened P waves, notched P waves, or both. T waves were flattened or inverted. Atrial arrhythmias were observed in one-third to two-thirds of patients, with atrial fibrillation accounting for 75% of these cases.
Echocardiography: Thickening or adhesion of the pericardium with enhanced echoes was evident. The left ventricular free wall showed a flattened appearance during mid-to-late diastole. Early rapid closure of the mitral valve and premature opening of the pulmonary valve were observed. Abnormal interventricular septal motion and reduced end-diastolic ventricular dimensions were also noted. Some consider rapid early diastolic ventricular filling as supporting evidence for the diagnosis of constrictive pericarditis. Abnormal dilation of the inferior vena cava was present.
X-ray examination: Chest radiographs revealed normal, slightly enlarged, or slightly reduced cardiac silhouettes. The cardiac contour appeared irregular and rigid. Widening of the superior mediastinum was attributed to dilation of the superior vena cava. The peripheral lung fields were clear. Pleural effusion was observed in 50–90% of patients, and unilateral pleural effusion without mediastinal shift was considered a significant sign of constrictive pericarditis. Pericardial calcification was a major radiographic finding, and its presence alongside clinical features confirmed the diagnosis. Extensive calcification was characteristic, with approximately 70% of patients showing calcification, commonly in the atrioventricular groove, the diaphragmatic and sternal surfaces of the right ventricle, and the left ventricular surface excluding the apex. Tomography also aided in identifying pericardial calcification.
CT and MRI: These modalities clearly demonstrated the extent of pericardial thickening, with a detection rate of around 80%. Ultrafast CT (UFCT) provided even greater accuracy. MRI was the best non-invasive method for diagnosing constrictive pericarditis, allowing precise measurement of pericardial thickness, as well as the degree of right atrial dilation and right ventricular reduction.
Cardiac catheterization: If non-invasive methods failed to confirm the diagnosis, right heart catheterization could be performed. Equalization of end-diastolic pressures in the right atrium, pulmonary artery, and left atrium was diagnostic. Right ventricular pressure showed a rapid early diastolic decline followed by a swift rise, then plateaued during mid-to-late diastole, termed the "square root sign," which further supported the diagnosis.
Laboratory tests: Some patients exhibited severe hypoalbuminemia and anemia. Isolated cases showed abnormal liver function and jaundice.
bubble_chart Treatment Measures
Constrictive pericarditis with significant clinical symptoms that do not improve after a period of treatment and rest generally has a poor natural prognosis. Most patients have difficulty regaining normal activity under conservative treatment. Somerville W proposed that once symptoms and signs of chronic constrictive pericarditis appear, the patient's life expectancy after losing general activity capacity is approximately 5 to 15 years. When ascites and other symptoms appear, the condition progresses rapidly, especially in children. Some patients eventually die from circulatory failure or hepatic and renal insufficiency. Therefore, once the diagnosis is confirmed, surgical intervention is the fundamental treatment, involving the removal of the constrictive pericardium to allow gradual recovery of cardiac function. Postoperative cardiac function recovery depends on: ① Choosing the appropriate timing for surgery, as it is easier to peel off the pericardium before fibrous calcification forms, and myocardial damage is also less severe; ② The extent of pericardial peeling, specifically whether the thickened pericardium on the surfaces of both ventricles can be completely removed. The surgery should be performed under relatively stable conditions. Thus, thorough and rigorous medical treatment should be administered preoperatively. For constrictive pericarditis caused by subcutaneous node bacteria, systematic anti-subcutaneous node drug therapy should be given, and surgery should be performed when body temperature, erythrocyte sedimentation rate (ESR), and overall nutritional status are near normal or relatively stable.
(I) Indications and Contraindications for Pericardial Stripping
1. Indications
(1) Surgery should be performed once the diagnosis of constrictive pericarditis is confirmed.
(2) For patients in poor condition—such as reduced food intake, severe ascites, impaired hepatic and renal function, low plasma protein, heart rate above 120 beats/min, and elevated ESR—conservative treatment should be administered first. Surgery should be scheduled once the condition stabilizes or improves.
(3) For severe cases with no significant improvement under conservative treatment, Hu Bingzhong and others advocate early pericardial fenestration to improve systemic function, followed by pericardiectomy.
2. Contraindications
(1) Elderly patients with severe cardiac or pulmonary diseases who cannot tolerate surgery.
(2) Patients with mild symptoms and no disease progression.
(II) Preoperative Preparation
1. Systemic supportive therapy, including dietary improvement, nutritional supplementation (low-salt and high-protein foods), vitamin supplementation, albumin infusion, and multiple small transfusions of fresh blood.
2. Unless confirmed as non-subcutaneous node constrictive pericarditis, anti-subcutaneous node therapy should be administered for no less than 6 weeks, preferably 3 months.
3. For patients with significant hepatomegaly, ascites, and peripheral edema, diuretics and potassium supplementation should be given as appropriate to correct typical edema and electrolyte imbalances.
4. For patients with excessive heart rate, small doses of digitalis may be administered as appropriate.
5. If pleural effusion and ascites remain significant after treatment, thoracentesis and paracentesis should be performed 1–2 days before surgery, with abdominal pressure bandaging to increase lung capacity and reduce intra-abdominal pressure, which facilitates diaphragmatic respiratory movement.
Preoperatively, the following goals should ideally be achieved: ① Significant improvement in circulatory and respiratory function; relief of dyspnea, orthopnea, edema, pleural effusion, and ascites; ② Improved dietary intake; ③ Heart rate below 120 beats/min, near-normal laboratory results, normal body temperature, and enhanced activity capacity; ④ Stable daily urine output.
(III) Surgical Approaches
Three common surgical approaches are used: ① Median sternotomy; ② Bilateral transverse thoracotomy; ③ Left anterolateral thoracotomy (Figure 1).
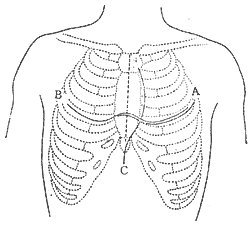
Figure 1 Three Common Incisions for Pericardiectomy
A. Left anterolateral incision; B. Bilateral transverse thoracotomy; C. Median sternotomy.
1. Median sternotomy incision. This surgical approach provides adequate exposure of the anterior and right lateral aspects of the heart, facilitating the dissection of thickened pericardium around the vena cava and right heart border, with minimal postoperative impact on respiratory function. It is commonly used for patients with concurrent pulmonary lesions or compromised respiratory function. The main disadvantage is poor exposure of the pericardium behind the left phrenic nerve and the cardiac apex. Some scholars argue that resection of the pericardium posterior to the phrenic nerve is unnecessary.
2. Left anterolateral thoracotomy: Enter the chest through the fifth intercostal space. On the right side, the internal thoracic artery needs to be ligated and divided, and the sternum transected. On the left side, the incision extends to the midaxillary line. The advantage of this approach is unilateral thoracotomy, which has minimal impact on respiratory function and is suitable for patients in poor condition. It provides good exposure of the left heart but poor exposure of the left ventricle and the superior and inferior vena cava.
3. Bilateral transverse thoracotomy: The advantage of this incision is excellent surgical field exposure, allowing access to both sides of the heart and complete pericardial resection. It also facilitates handling unexpected intraoperative complications. The disadvantage is the longer incision, greater trauma, and significant postoperative impact on pulmonary function.
4. Pericardiectomy via left anterolateral thoracotomy: After anesthesia, the patient is placed in a supine position with a pillow under the left scapula and the left arm positioned beneath the side (Figure 2). A curved incision is made along the fifth intercostal space below the left breast. The muscles are incised to enter the chest. The internal thoracic artery is ligated and divided. The fifth costal cartilage is transected near the sternum. The chest is retracted to expose the thoracic cavity. The left phrenic nerve is sharply dissected from the pericardium, preserving as much fat and soft tissue as possible to avoid nerve injury. The pericardium is incised over the left ventricle, preferably posterolaterally, in a non-calcified area. Below the incision, layers or pericardial effusion may sometimes be seen. However, in most cases, the incision reaches the myocardial surface directly. A plane is identified outside the epicardium, and blunt or sharp dissection is performed along this plane to gradually expand the area. If a space remains between the thickened parietal and visceral pericardium, the parietal layer can be excised first to improve cardiac pulsation, followed by addressing the fibrotic visceral pericardium. If adhesions are dense or the plane is unclear, scissors or a scalpel should be used for sharp dissection, proceeding meticulously to avoid myocardial injury or rupture. Avoid forceful blunt dissection to prevent myocardial trauma or rupture.
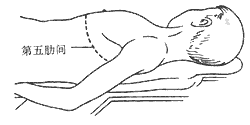
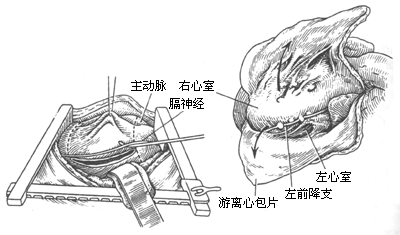
Figure 2: Schematic diagram of the left anterolateral thoracotomy procedure.
The order of pericardial dissection should proceed along both sides of the longitudinal incision, separating toward the right anterior and left posterior directions. En bloc resection is preferred. If myocardial rupture occurs, the already dissected pericardium can be used for repair and hemostasis. The right pericardial flap should be dissected to the left atrioventricular groove, with the superior boundary below the thymus. The left pericardial flap should be dissected superiorly to the main pulmonary artery, and its constricting ring should be transected to prevent severe postoperative right ventricular hypertension. The inferior boundary should completely free or excise the thickened pericardium beyond the diaphragmatic tendon. The posterior boundary should free as much pericardium as possible from the left ventricular surface. Special care must be taken to avoid injury to coronary artery branches when dissecting the interventricular groove. If calcification is present in this area, it should be left in place, and dissection should continue elsewhere. The thickened pericardium over the left atrium has minimal hemodynamic impact, is prone to tearing during dissection, and is difficult to control for bleeding; thus, aggressive dissection is unnecessary. The constricting rings near the left atrium and inferior vena cava should be incised and released. Any constricting rings in the atrioventricular groove should be transected. Intraoperatively, the pericardium over the left ventricle should be completely dissected first, followed by the right ventricular outflow tract, to prevent acute pulmonary edema. After complete pericardial dissection, the pericardial flaps are excised. In severe cases, myocardial atrophy is evident. After pericardial dissection, the myocardial surface may appear pale, and the extent of dissection should be moderate, focusing on relieving ventricular surface constrictions. Digitalis preparations can be administered post-dissection. Meticulous hemostasis is essential at the end of the procedure, with electrocautery applied to the pericardial resection edges. If necessary, pressure-monitoring catheters can be placed in the left atrial appendage or pulmonary veins for postoperative monitoring. Two closed thoracic drainage tubes are placed.
5. Pericardiectomy via Median Sternotomy General anesthesia with endotracheal intubation is employed. The patient is placed in a supine position with the scapular region elevated to protrude the chest, followed by median sternotomy. If retrosternal adhesions are present, they should be dissected while gradually retracting the sternum with a sternal retractor. The pericardial dissection begins at the cardiac apex. Here, the pericardial adhesions are typically mild, with no significant thickening, making dissection easier. The thickened pericardium is sequentially incised with a scalpel. A layer of loose connective tissue often exists between the thickened pericardium and the outer membrane, serving as the correct dissection plane. After incising the thickened pericardium, the pulsating heart can be seen bulging outward. Once a portion of the pericardium is separated, the assistant gently lifts the pericardial flap with forceps while the surgeon lightly presses the heart surface with the left hand to fully expose the extent of adhesion between the thickened pericardium and the myocardium. For loose adhesions, blunt dissection can be performed using a gauze-wrapped finger or a peanut dissector, applying force on the pericardial surface. For band-like adhesions, sharp dissection with scissors or a scalpel is required. If adhesions are extremely dense, the original dissection site should be abandoned, and a new incision should be made elsewhere to separate the pericardium—prioritizing easier areas first. The extent of dissection is determined by the patient's intraoperative cardiac function and the degree of pericardial adhesion. The basic dissection scope generally includes: complete removal at the cardiac apex; the left side near the left phrenic nerve; and release of the fibrotic constriction rings at the atrioventricular groove and the inferior vena cava entrance. The sequence of dissection should be: left ventricle → right ventricular outflow tract → atrioventricular groove constriction ring → inferior vena cava annular band.
If the pericardium is well-organized and very easy to peel, complete pericardial stripping is optimal. If arrhythmias, circulatory instability, pale myocardium, cardiac enlargement, or weakened myocardial contraction occur during the procedure, the peeling operation should be stopped appropriately, with only the main areas (the left and right ventricular surfaces and the constriction ring of the inferior vena cava) being stripped. Digoxin and diuretics should be administered simultaneously, and the surgery should be completed as early as possible to enhance safety. Postoperatively, positive inotropic drugs such as dopamine should be given if necessary.
(IV) Surgical Complications and Preventive Measures
1. Low Cardiac Output: During pericardial stripping, acute cardiac dilation, especially after peeling the pericardium on the right ventricular surface, can lead to rapid ventricular filling and expansion due to systemic venous hypertension, resulting in acute low cardiac output. Therefore, intraoperative fluid intake should be restricted. After relieving left ventricular constriction, cedilanid and furosemide should be administered immediately to strengthen the heart while eliminating excess fluid and reducing cardiac burden. Within 12–48 hours postoperatively, dopamine and other catecholamine drugs should be used. If the response to medication is poor and low cardiac output cannot be corrected, intra-aortic balloon counterpulsation may be employed.
2. Phrenic Nerve Injury: Before starting pericardial stripping via a left anterolateral incision, Kirklin JW suggested first mobilizing the left phrenic nerve and preserving as much fat and soft tissue around it as possible. Injury to the phrenic nerve can cause paradoxical diaphragmatic breathing, impairing gas exchange and hindering the expulsion of respiratory secretions.
3. Coronary Artery Injury: Special care must be taken when dissecting the anterior interventricular groove to avoid injuring the coronary artery. Bleeding from its branches or terminals can be stopped by ligation. If localized calcified plaques are encountered in this area, they may be left in place rather than forcibly removed.
4. Myocardial Rupture: For calcified lesions embedded in the myocardium, they can generally be left as "islands" and should not be forcibly peeled. If the boundaries of the peeling are unclear or severe adhesions are present, the thickened pericardium can be incised in a "grid" pattern to partially relieve myocardial surface constriction. In the event of myocardial rupture, the surgeon should press the index finger of the left hand flat over the tear and suture a free pericardial patch around the rupture site to save the patient’s life.
(V) Postoperative Management
1. General Care: Routine oxygen administration is required, with close monitoring of blood pressure, respiration, pulse, heart rate, and urine output. Ensure the drainage tube remains unobstructed. If bleeding is excessive, blood transfusion may be considered.
2. Prophylactic Antibiotics: In addition to routine antibiotics, patients with tuberculous pericarditis should receive standard anti-tuberculosis therapy for six months to one year postoperatively.
3. Cardiac Support and Diuresis: Continue administering diuretics postoperatively to reduce sodium and water retention. Digitalis preparations should be given under adequate potassium supplementation, and fluid intake should be strictly controlled.
Surgical Outcomes
1. Surgical Mortality: In recent years, the mortality rate has declined to approximately 4%. McCaughan BC reported that the patient’s preoperative cardiac functional status is the most critical factor affecting surgical mortality. For patients with NYHA Class I–II cardiac function, the surgical mortality rate is 0%; for Class III and IV, the rates are 10% and 46%, respectively. Preoperative ascites, peripheral edema, intracardiac pressure, and the degree of low cardiac index also influence surgical mortality.
2. Long-Term Survival: Kirklin JW reported 5-year and 15-year survival rates of 84% and 59%, respectively. McCaughan BC reported 5-year, 15-year, and 30-year survival rates of 84%, 71%, and 52%, respectively. The primary factor affecting long-term survival remains preoperative cardiac function, with no significant correlation to the surgical approach (Figure 3).
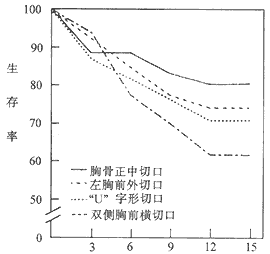
Postoperative Follow-Up Duration (Years)
Figure 3: Long-Term Survival Rates After Pericardial Stripping
In addition, Kirklin reported that using a median sternotomy and left anterolateral incision resulted in a reoperation rate of only 2%.
3. Postoperative Hemodynamic Changes All patients showed normal hemodynamic parameters of cardiac function at rest. Approximately 10-20% of patients exhibited a slight increase in pulmonary artery pressure during physical activity, with no compensatory rise in cardiac output. If incomplete pericardiectomy occurs due to thickened pericardium on the ventricular surface, hemodynamic improvement may be suboptimal. McCaughan BC reported favorable long-term outcomes in most patients, with nearly all achieving NYHA functional class I-II (Figure 4).
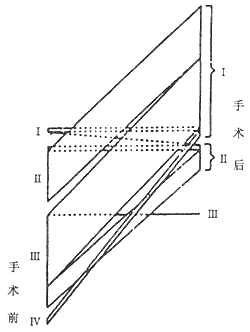
Figure 4 Schematic diagram of preoperative and postoperative cardiac function changes in 141 patients
Li Fayin et al. reported 132 cases of constrictive pericardiectomy with a surgical mortality rate of 3%. Among 99 patients followed for 1-25 years (average 8.2 years), the cure rate was 73%, symptom improvement occurred in 22%, no significant improvement in 3%, and advanced stage recurrence mortality in 2%. They suggested that pericardial resection should extend approximately 1 cm anterior to the phrenic nerves bilaterally. Early surgery was emphasized: patients with disease duration <1 year had an 80% cure rate versus only 52% in those with >1 year duration. Four of the five surgical deaths occurred in patients with disease duration exceeding one year.
Pericardiectomy should be performed as early as possible, with most patients achieving satisfactory results. A prolonged course of the disease may lead to myocardial atrophy and cardiac cirrhosis, resulting in a poorer prognosis. Without surgical treatment, the condition may worsen, and a small number of cases may suffer long-term illness, severely limiting their daily life and work.
Based on clinical manifestations such as dyspnea, hepatomegaly, ascites, elevated venous pressure, decreased pulse pressure, paradoxical pulse, and peripheral pitting edema, combined with auxiliary methods like X-ray, electrocardiogram, and echocardiography, a correct diagnosis can generally be made.
The main diseases that need to be differentiated from this condition include the following:
1. Congestive heart failure: A history of heart disease, cardiac enlargement, and often the presence of cardiac valve murmurs, with significant lower limb edema but relatively mild abdominal distension and fullness. After diuretic use, venous pressure drops significantly, whereas in chronic constrictive pericarditis, diuretics have little effect on venous pressure.
2. Portal hypertension due to cirrhosis or hepatic vein thrombosis: Both may present with hepatomegaly and/or ascites. Differentiation from constrictive pericarditis is straightforward based on clinical symptoms and whether venous pressure in the head and upper limbs is elevated. Additionally, barium swallow examination in patients with portal hypertension may reveal varices in the lower esophagus.
3. Primary cardiomyopathy: In patients with dilated cardiomyopathy, physical examination may reveal significant cardiac enlargement, displacement of the apical impulse to the left, and systolic murmurs of the mitral or tricuspid valve on auscultation. ECG may show left ventricular hypertrophy, left bundle branch block, or pathological Q waves and T-wave inversion. X-ray may demonstrate cardiac enlargement bilaterally, especially in the left ventricle, with weakened pulsations and no obvious dilation of the superior vena cava. The hemodynamic changes and clinical manifestations of right ventricular or biventricular restrictive cardiomyopathy are quite similar to those of constrictive pericarditis. However, echocardiography in restrictive cardiomyopathy may reveal characteristic thickening and increased reflectivity of the myocardium and endocardium, reduced ventricular cavity size, and apical occlusion, which can aid in differentiation. In a few cases where the diagnosis remains uncertain after comprehensive examination, a repeat pericardial biopsy may be performed. An incision is made through the left fifth intercostal space to remove a piece of pericardium for pathological examination. If constrictive pericarditis is confirmed, the original incision can be extended to perform a pericardiectomy.
4. Tricuspid stenosis: This condition is characterized by its distinctive murmur and associated valve damage (aortic and mitral valves), absence of early diastolic collapse in the jugular vein, and Doppler ultrasound detection of a diastolic transvalvular pressure gradient across the tricuspid valve. When accompanied by tricuspid regurgitation, it may produce systolic jugular venous pulsations, hepatic pulsations, and a pansystolic murmur.




