| disease | Intestinal Cancer |
Intestinal cancer is a common malignant tumor in the gastrointestinal tract, with the highest incidence in the 40-50 age group. The cause of the disease is not yet fully understood, but some conditions such as familial polyposis have been recognized as precancerous lesions; colon adenomas, ulcerative colitis, and colonic schistosomiasis granulomas are closely related to the occurrence of intestinal cancer.
bubble_chart Clinical Manifestations
Most patients with colon cancer are middle-aged or older, with a median age of 45, and about 5% of patients are under 30 years old. The clinical manifestations of colon cancer vary depending on the size, location, and pathological type of the lesion. Many early-stage colon cancer patients may have no symptoms clinically, but as the disease progresses and the lesion continues to grow, a series of common symptoms of colon cancer can arise, such as increased frequency of bowel movements, blood and mucus in the stool, abdominal pain, diarrhea or constipation, intestinal obstruction, as well as systemic symptoms like lack of strength, weight loss, and anemia. The entire colon is divided into two parts by the middle of the transverse colon: the right colon and the left colon. The clinical manifestations of cancer in these two parts have their own characteristics, which are described as follows:
(1) Right colon cancer The right colon has a large lumen, and the feces in this part of the intestine are liquid. The cancer in this segment is mostly ulcerative or cauliflower-like protruding into the intestinal lumen, rarely causing annular stenosis, so obstruction is uncommon. However, these cancers often ulcerate and bleed, leading to secondary infection and toxin absorption, resulting in clinical symptoms such as abdominal discomfort, changes in bowel habits, abdominal mass, anemia, weight loss, or cachexia.
1. Abdominal discomfort About 75% of patients experience abdominal discomfort or dull pain, initially intermittent and later becoming continuous, often located in the lower right abdomen, resembling chronic appendicitis. If the tumor is located at the hepatic flexure and the stool is dry, colicky pain may also occur, which should be differentiated from chronic cholecystitis. About 50% of patients experience loss of appetite, bloating, belching, nausea, and vomiting.2. Changes in bowel habits Early-stage stools are thin, with pus and blood, and increased frequency of bowel movements, related to the formation of cancer ulcers. As the tumor grows and affects stool passage, diarrhea and constipation may alternate. The amount of bleeding is small, and as the stool mixes with the blood due to colonic peristalsis, it may not be visible to the naked eye, but the occult blood test is often positive.
3. Abdominal mass More than half of the patients can be found to have an abdominal mass upon consultation. This mass may be the cancer itself or a lump formed by extraintestinal infiltration and adhesion. The former has a more regular shape and clear outline, while the latter is less regular. The mass is generally hard, and once secondary infection occurs, it becomes less mobile and tender.
4. Anemia and cachexia About 30% of patients develop anemia due to continuous bleeding from the ulcerated cancer, along with weight loss, weakness in the limbs, and even systemic cachexia.
(2) Left colon cancer The left colon has a narrower lumen, and the feces become dry and hard as water is absorbed. Left colon cancer is mostly infiltrative, often causing annular stenosis, so the clinical manifestations are mainly acute or chronic intestinal obstruction. The tumor volume is smaller, with less ulceration and bleeding, and no toxin absorption, so anemia, weight loss, cachexia, etc., are rare, and the mass is not easily palpable.
1. Abdominal colicky pain This is the main manifestation of cancer with intestinal obstruction. The obstruction can occur suddenly, with abdominal colicky pain, accompanied by abdominal distension and fullness, hyperactive bowel sounds, constipation, and obstructed gas passage; chronic obstruction manifests as abdominal distension and fullness discomfort, paroxysmal abdominal pain, hyperactive borborygmi, constipation, blood and mucus in the stool, and partial intestinal obstruction may persist for months before turning into complete obstruction.2. Difficulty in defecation Half of the patients have this symptom, and as the disease progresses, constipation becomes more severe. If the cancer is located lower, there may also be a feeling of incomplete defecation and tenesmus.
3. Blood or mucus in the stool As the feces in the left colon gradually become formed, blood and mucus do not mix with the stool, and about 25% of patients have visible fresh blood and mucus in their stool upon naked-eye observation.
Most patients with colon cancer are middle-aged or older, with a median age of 45, and about 5% of patients are under 30 years old. The clinical manifestations of colon cancer vary depending on the size, location, and pathological type of the lesion. Many early-stage colon cancer patients may have no symptoms clinically, but as the disease progresses and the lesion enlarges, a series of common symptoms of colon cancer can arise, such as increased frequency of bowel movements, bloody and mucous stools, abdominal pain, diarrhea or constipation, intestinal obstruction, as well as systemic symptoms like lack of strength, weight loss, and anemia. The entire colon is divided into the right and left halves by the middle of the transverse colon, and the clinical manifestations of cancers in these two parts have their own characteristics, which are described as follows:
(1) Right-sided colon cancer The right colon has a large lumen, and the feces inside are liquid. The cancers in this part of the intestine are mostly ulcerative or cauliflower-like cancers protruding into the intestinal cavity, rarely causing annular stenosis, so obstruction is uncommon. However, these cancers often ulcerate and bleed, leading to secondary infections and toxin absorption, resulting in clinical symptoms such as abdominal discomfort, changes in bowel habits, abdominal masses, anemia, weight loss, or cachexia.1. Abdominal discomfort About 75% of patients experience abdominal discomfort or dull pain, initially intermittent and later becoming continuous, often located in the lower right abdomen, resembling chronic appendicitis. If the tumor is located at the hepatic flexure and the feces are dry, colicky pain may also occur, which should be differentiated from chronic cholecystitis. About 50% of patients experience loss of appetite, bloating, belching, nausea, and vomiting.
2. Changes in bowel habits Early-stage symptoms include thin stools with pus and blood, and increased frequency of bowel movements, related to the formation of cancerous ulcers. As the tumor enlarges and affects the passage of feces, alternating diarrhea and constipation may occur. The amount of bleeding is small, and as it mixes well with feces due to colonic peristalsis, it is not easily visible to the naked eye, but occult blood tests are often positive.
3. Abdominal masses More than half of the patients can be found to have abdominal masses upon consultation. These masses may be the cancer itself or a lump formed by extramural infiltration and adhesion. The former has a more regular shape and clear outline, while the latter is less regular. The masses are generally hard, and once secondary infection occurs, their movement is restricted, and they are tender.
4. Anemia and cachexia About 30% of patients develop anemia due to continuous bleeding from the ulcerated cancer, along with weight loss, weakness in the limbs, and even systemic cachexia.
(2) Left-sided colon cancer The left colon has a narrower lumen, and the feces inside become dry and hard as water is absorbed. Most left-sided colon cancers are infiltrative, often causing annular stenosis, so the main clinical manifestations are acute or chronic intestinal obstruction. The tumor volume is smaller, with less ulceration and bleeding, and no toxin absorption, so anemia, weight loss, cachexia, etc., are rare, and it is also difficult to palpate the mass.
1. Abdominal colicky pain This is the main manifestation of cancer accompanied by intestinal obstruction. The obstruction can occur suddenly, with abdominal colicky pain, accompanied by abdominal distension and fullness, hyperactive bowel sounds, constipation, and obstructed gas passage; chronic obstruction is manifested as abdominal distension and fullness discomfort, paroxysmal abdominal pain, hyperactive borborygmi, constipation, bloody and mucous stools, and partial intestinal obstruction sometimes lasts for several months before turning into complete intestinal obstruction.
2. Difficulty in defecation Half of the patients have this symptom, and as the disease progresses, constipation becomes more severe. If the cancer is located lower, there may also be a feeling of incomplete defecation and tenesmus.
3. Blood or mucus in stools As the feces in the left colon gradually become formed, blood and mucus do not mix with the feces, and about 25% of patients can see fresh blood and mucus in their stools with the naked eye.
Diagnosis: The early symptoms of intestinal cancer are often mild or not obvious, and are frequently overlooked by patients, making it easy to misdiagnose as fistula disease. Therefore, for patients over middle age, the following manifestations should raise vigilance and prompt consideration of the possibility of intestinal cancer: ① Recent changes in bowel habits (such as constipation, diarrhea, or difficulty in defecation), persistent abdominal discomfort, dull pain, or abdominal distension and fullness; ② Stools become loose, or contain blood and mucus; ③ Persistent positive fecal occult blood test; ④ Unexplained anemia, lack of strength, or weight loss; ⑤ A palpable abdominal mass.
When the aforementioned suspicious phenomena are observed, in addition to further history inquiry and physical examination, the following systematic examinations should be immediately conducted to confirm the diagnosis.
(1) Digital rectal examination and proctoscopy: To check for the presence of rectal tumors, rectal cancer, internal hemorrhoids, or other lesions for differential diagnosis.
(2) Sigmoidoscopy and fiber colonoscopy: Although the sigmoidoscope is only 25 cm long, 75% of large intestine cancers are located within the range that can be observed by sigmoidoscopy. During the examination, not only can the cancer be detected, but its size, location, and local infiltration range can also be observed. Tissue can be taken through the sigmoidoscope for pathological examination. Fiber colonoscopy provides a higher diagnostic rate and has been widely used domestically. Skilled operators can insert the fiber colonoscope into the cecum and terminal ileum, and can also take photographs, making it an ideal examination method.
(3) X-ray examination
1. Abdominal plain film examination: Suitable for cases accompanied by acute intestinal obstruction, showing gas-filled and enlarged colon above the obstruction site.
2. Barium enema examination: Can reveal rigid intestinal walls at the cancer site, poor expansibility, weakened or disappeared peristalsis at the lesion site, irregular or disappeared colonic haustra, narrowed intestinal lumen, disordered, destroyed, or disappeared mucosal folds, and filling defects. Barium-air double contrast radiography is more helpful in diagnosing pedunculated tumors in the colon.
(4) Carcinoembryonic antigen (CEA) test: Has little diagnostic value for early cases but is somewhat helpful in predicting prognosis and judging recurrence.
Currently, there is no practical and feasible plan for large-scale population prevention and screening of colorectal cancer. Only when the aforementioned suspicious signs of colorectal cancer are encountered, appropriate examination methods should be promptly selected for early diagnosis, especially for those with positive fecal occult blood tests, and the cause must be further investigated.
bubble_chart Treatment Measures
The best treatment for intestinal cancer is still complete surgical resection after early diagnosis.
(1) Surgical Treatment
1. Radical Surgery The scope of surgical resection should include the intestinal segment where the cancer is located and the regional lymph nodes near the supplying arteries. There are different surgical methods as follows:
Right-sided intestinal cancer: Right hemicolectomy is performed, including the cecum, ascending colon, hepatic flexure, the right half of the transverse colon, the terminal ileum (10 cm), and the related mesentery and lymph nodes (Figure 1). Then, an end-to-end or end-to-side anastomosis is performed between the terminal ileum and the transverse colon. If the cancer is located in the cecum, the ileocolic artery and the right colic artery should be ligated and cut at their branching points on the mesentery, and only the right branch of the middle colic artery should be ligated. If the cancer is located at the hepatic flexure, the middle colic artery must be ligated and cut at its origin to completely remove the related lymph nodes near the artery.
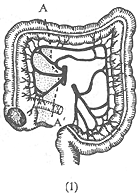
(1) A-A' shows the resection range for cecal and ascending colon cancer.
(2) B-B' shows the resection range for hepatic flexure cancer.
Figure 1: Right Hemicolectomy
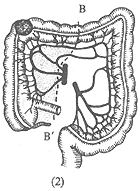
Transverse colon cancer: Transverse colon resection and end-to-end anastomosis of the two cut ends, with single ligation and cutting of the middle colic artery (Figure 2). The resection range should include the entire transverse colon, the gastrocolic mesentery, the greater omentum, and the lymph nodes near the root of the middle colic artery, followed by end-to-end anastomosis of the ascending and descending colon cut ends.
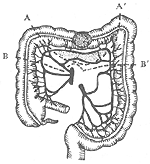
Figure 2: Transverse Colon Resection
A-A' shows the resection range for transverse colon cancer.
B-B' shows the extended resection range for transverse colon cancer.
Left-sided intestinal cancer: Left hemicolectomy is performed, including the left half of the transverse colon, the splenic flexure, the descending colon, and part of the sigmoid colon, followed by end-to-end anastomosis of the transverse colon and sigmoid colon. However, the resection range should be slightly adjusted according to the location of the cancer (Figure 3). If the cancer is located at the splenic flexure, the left branch of the middle colic artery and the left colic artery should be ligated. If the cancer is located in the descending colon, the first branch of the sigmoid colon artery at the top should also be ligated and cut, and the corresponding lymph nodes near the artery should be cleared.
(1) A-A' shows the resection range for splenic flexure cancer.
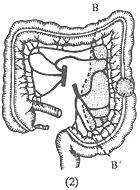
(2) B-B' shows the resection range for descending colon cancer.
Figure 3: Left Hemicolectomy
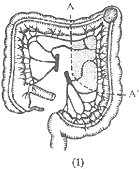
Sigmoid colon cancer: The resection range for radical surgery depends on the length of the sigmoid colon and the location of the cancer. If the cancer is located in the upper segment of the sigmoid colon, part of the descending colon and the sigmoid colon are resected (Figure 4). If the cancer is near the sigmoid-rectal junction, most of the rectum should also be resected, and an end-to-end anastomosis of the descending colon and rectum is performed.
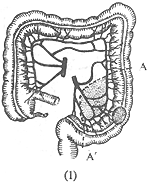
(1) A-A' shows the resection range for upper sigmoid colon cancer.
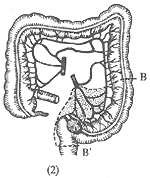
(2) B-B' shows the resection range for cancer at the sigmoid-rectal junction.
Figure 4: Surgical Resection for Sigmoid Colon Cancer
When intestinal cancer is accompanied by significant obstruction, staged surgery is generally required. Initially, a stoma is created above the obstructed tumor for decompression, such as a transverse colostomy or sigmoid colostomy. Once the patient's condition improves and the intestinal wall inflammation and edema subside, typically 2-3 weeks after the stoma surgery, an intermediate stage [second stage] radical tumor resection is performed.
2. Palliative Surgery: When intestinal cancer has metastasized to the liver or distant sites, or when the cancer has extensively infiltrated locally and cannot be radically treated, the following principles can be applied:
If the local lesion of the intestinal cancer is still resectable, a palliative resection should be attempted to alleviate symptoms. Postoperative adjuvant anticancer therapy can prolong survival.
If the lesion is extensively infiltrated and fixed and cannot be resected, a bypass anastomosis can be performed on the intestinal segments proximal and distal to the cancer site, such as a side-to-side anastomosis between the terminal ileum and the transverse colon in right-sided intestinal cancer.
3. Preoperative Bowel Preparation: This is extremely important, aiming to empty the colon and reduce the bacterial load in the intestinal lumen to prevent postoperative infections. There are two common methods:
One method involves oral administration of intestinal antibacterial drugs, laxatives, and multiple enemas. The patient should consume a liquid diet for 2 days before surgery and undergo a cleansing enema the night before surgery. The other method is total bowel irrigation, which starts at noon the day before surgery by switching to a liquid diet. Irrigation begins 4 hours after lunch, using a solution of 6g sodium chloride, 2.5g sodium bicarbonate, and 0.75g potassium chloride in 1000ml of warm water at around 37°C. The irrigation fluid is infused through a nasogastric tube inserted into the stomach, starting at a rate of 3000-4000ml per hour, then slowing to 2000-3000ml per hour until the fluid expelled from the anus is clear and free of fecal matter. The entire process takes about 3 hours, and the total volume of irrigation fluid should not be less than 6000ml. If the patient feels hungry after irrigation, chocolate or sugar water can be given before bedtime.
(2) Chemotherapy: For patients who cannot undergo radical surgery, have postoperative recurrence, and cannot undergo further surgery, chemotherapy is a primary treatment option. Combined radiotherapy and surgery can reduce local recurrence, and postoperative chemotherapy can help control potential hematogenous metastases.
1. Chemotherapeutic Agents
(1) Fluorouracil (5-Fu): The most commonly used, administered intravenously at 12mg/kg daily for 5 days, then halving the dose and administering every other day until toxic reactions such as diarrhea, gastritis, nausea, vomiting, and leukopenia occur. Outpatients can follow a regimen of 15-20mg/kg once a week. Oral administration is less effective, with 20mg/kg daily for 5 days, repeated every 5 weeks. Fluorouracil can also be used for intraoperative intestinal perfusion, where the intestinal segment above and below the cancer is ligated with gauze, and 30mg/kg of fluorouracil is injected into this segment, followed by resection 30 minutes later. Recent reports indicate that rectal administration of fluorouracil results in higher drug concentrations in the cancer tissue compared to intravenous administration. Intraperitoneal perfusion of fluorouracil also shows good efficacy.
(2) Mitomycin: Administered once or twice a week, 4-6mg dissolved in 20-40ml of saline, intravenously, with a total dose of 40-60mg per course. Some advocate for intravenous administration once every 3-4 weeks, 20mg each time.
(3) Nitrosoureas: Carmustine (BCNU) at 90-125mg/m2 daily, dissolved in 250ml of 5% glucose or saline, administered intravenously over 2-3 days per course. Lomustine (CCNU) at 130mg/m2 orally, once every 6-8 weeks, with 3 doses per course. Semustine (Me-CCNU) at 200-220mg/m2 orally, once every 6-8 weeks, with 3 doses per course.
(4) Tegafur (FT207): Its effect is similar to that of fluorouracil, but its toxicity is 3 to 4 times lower. The daily dose is 800 to 1000 mg, divided into 3 to 4 oral doses, and it can also be administered intravenously, with 20g constituting one treatment course. It has cross-resistance with fluorouracil, meaning that if fluorouracil is ineffective, switching to FT207 will also be ineffective.
2. Combination Chemotherapy The combination chemotherapy regimen for large intestine cancer is still under exploration, with the basic regimen still being fluorouracil and semustine. For example, semustine 100mg/m2, taken orally once a month; fluorouracil 10mg/kg, administered intravenously once a day for 5 days, repeated every 5 weeks; vincristine 1mg/m2 administered intravenously, used once on the first day, repeated weekly.
(3) Radiotherapy Not sensitive, generally not used.
The Tumor Hospital affiliated with Shanghai Medical University followed up 324 patients who underwent radical surgery for intestinal cancer until June 1983. The 5-year and 10-year survival rates were 54.63% and 53.90%, respectively. The prognosis was clearly related to the size of the tumor, pathological type, and clinical stage. The median survival time for patients who underwent palliative resection for intestinal cancer was 11 months.




