| disease | Congenital Aortic Coarctation |
Coarctation of the aorta accounts for approximately 5-8% of all congenital heart diseases. In 1760, Morgagni first identified this condition during an autopsy. The primary pathology involves localized narrowing or occlusion of a short segment of the aorta, leading to impaired blood flow. In the vast majority of cases (over 95%), the coarctation occurs at the distal segment of the aortic arch and the junction with the descending thoracic aorta, known as the aortic isthmus, near the ductus arteriosus or ligamentum arteriosum. However, in rare instances, the narrowing may be located in the aortic arch, descending thoracic aorta, or even the abdominal aorta. Occasionally, the aorta may exhibit coarctation at two sites. A family history of this condition is extremely uncommon. It is more prevalent in males, with a male-to-female ratio of 3-5:1.
bubble_chart Etiology
The mechanism of disease causing the narrowing of the main stirred pulse is still unclear. Craigil and sklda once believed that the most common narrowing of the main stirred pulse at the isthmus is caused by the contraction of smooth muscle and fibrous tissue in the wall of the stirred pulse duct during its closure, which affects the wall of the main stirred pulse at the isthmus, leading to narrowing. In recent years, Ho et al. conducted histological examinations on 35 specimens through continuous sectioning and confirmed that the stirred pulse duct tissue completely surrounds the descending main stirred pulse near the duct, forming a structurally continuous passage. However, this theory cannot explain cases where the narrowing of the main stirred pulse coexists with a non-closed stirred pulse duct or where the narrowing occurs far from the stirred pulse duct area. Over the past two decades, many scholars have proposed that an imbalance in blood flow between the main stirred pulse and the pulmonary stirred pulse during the fetal period is the primary disease cause of main stirred pulse narrowing. Under normal circumstances, the output of the left and right ventricles during the fetal period is roughly equal. Blood returning through the superior vena cava is pumped by the right ventricle to the lungs and enters the descending main stirred pulse via the stirred pulse duct. Blood returning from the inferior vena cava to the heart enters the left atrium through the foramen ovale, transmission from one meridian to the next, and is pumped by the left ventricle to the coronary stirred pulse and the brachiocephalic stirred pulse. Only 30% of the blood ejected by the left ventricle enters the descending main stirred pulse through the isthmus, merging with blood from the stirred pulse duct. Rudolph observed blood flow in fetal lambs and found that the blood flow through the isthmus of the main stirred pulse was only about half of that through the stirred pulse duct. Therefore, the diameter of the main stirred pulse isthmus during the fetal period is generally smaller than that of the ascending and descending main stirred pulse. If the blood flow ejected by the left ventricle decreases during the fetal period, the blood flow in the main stirred pulse decreases, while the blood flow in the pulmonary stirred pulse increases accordingly. Reduced blood flow through the isthmus of the main stirred pulse can lead to narrowing or even occlusion of the isthmus. If the foramen ovale is small and blood flow resistance increases, a large amount of blood returning from the inferior vena cava to the right atrium enters the right ventricle, increasing blood flow through the stirred pulse duct while reducing blood flow through the left ventricle and the isthmus of the main stirred pulse, promoting the formation of main stirred pulse narrowing. In severe cases of reduced blood flow, the isthmus of the main stirred pulse may exhibit hypoplasia. Narrowing of the main stirred pulse, hypoplasia of the ascending main stirred pulse, and conditions such as ventricular septal defects causing left-to-right shunting can also lead to reduced blood flow in the main stirred pulse and increased blood flow in the pulmonary stirred pulse, resulting in narrowing or hypoplasia of the isthmus of the main stirred pulse.
The most common site of aortic coarctation is at the aortic duct or the adjacent portion where the aortic ligament connects to the main aortic artery. The external contour of the coarctation segment of the aorta is inwardly concave, but the depression of the aortic wall at the attachment site of the aortic ligament is not obvious, or may even slightly protrude. The boundaries between the coarctation segment and its adjacent areas are distinct, typically measuring less than 1 cm in length. The distal segment of the aortic arch connected to the proximal end of the coarctation gradually tapers, forming a conical shape. The descending aorta connected to the distal end of the coarctation may exhibit an enlarged external diameter and thickened vessel wall. The internal diameter of the coarctation segment is often smaller than its external appearance, with thickening of the aortic wall's middle layer protruding into the aortic lumen, forming a septum or membrane. The intimal layer of the aortic wall is also hypertrophied. The aortic lumen is narrow, allowing only the passage of a probe or measuring just a few millimeters, located either centrally or eccentrically within the septal membrane. The aortic wall distal to the coarctation segment often exhibits intimal thickening due to the impact of blood flow. The heart is usually enlarged, with left ventricular hypertrophy being very common. The middle layer of the coronary arteries is often thickened, reducing the lumen, which may lead to symptoms of insufficient coronary circulation earlier. In about 25–40% of cases, the aortic valve is bicuspid. The intercostal arteries are significantly enlarged, with abundant collateral circulation in the chest wall. In a minority of cases, branches of the aortic arch may also exhibit abnormalities, such as stenosis of the left subclavian artery, stenosis of the right subclavian artery, or ectopic origin of the right subclavian artery from the proximal or distal end of the aortic coarctation. Due to elevated blood pressure proximal to the coarctation, rich collateral circulation, and enlarged, tortuous arteries, aneurysms are prone to form in the intracranial arteries, the aorta proximal and distal to the coarctation, and the intercostal arteries. The incidence of such aneurysms increases with age. Rupture of an aneurysm can be fatal.
The vast majority of aortic coarctations occur at the aortic isthmus. Based on the anatomical relationship between the coarctation segment and the aortic ligament or duct, they can be classified into two types: preductal and postductal (Figure 8).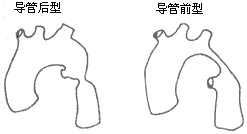
Figure 8 Types of Aortic Coarctation
Preductal aortic coarctation: The coarctation segment is located proximal to the aortic ligament or duct, and this type is relatively rare. The coarctation segment may be longer. In most cases, the ductus arteriosus remains patent. In severe cases, blood ejected by the right ventricle enters the descending aorta via the pulmonary artery and the patent ductus arteriosus to supply the lower half of the body, with less developed collateral circulation. Nearly half of preductal aortic coarctation cases are associated with other congenital cardiovascular anomalies, which can lead to fatal heart failure during infancy. Therefore, Bonnet historically referred to this type as infantile aortic coarctation.
Postductal aortic coarctation: This type is relatively common. In typical cases, the coarctation segment is located in the aortic isthmus distal to the origin of the left subclavian artery, and in most cases, the ductus arteriosus has closed. The coarctation lesion is short and localized, situated distal to or adjacent to the ligamentum arteriosus. The aorta proximal and distal to the coarctation often exhibits varying degrees of dilation. A rich collateral circulation forms between the aorta proximal and distal to the coarctation (Figure 9). In cases where the ductus arteriosus remains patent, the direction of blood flow through the ductus depends on the pressure difference between the descending aorta and the pulmonary artery. Approximately 25–40% of postductal aortic coarctation cases present with a bicuspid aortic valve, but they generally do not involve other severe congenital cardiovascular malformations, and most patients can survive into adulthood. Therefore, Bonnet historically referred to this type as adult-type aortic coarctation. In postductal aortic coarctation cases, collateral circulation between the aorta proximal and distal to the coarctation begins to form during the fetal period to enhance blood supply to the distal segment of the stenosis. Cases with severe coarctation and a closed ductus arteriosus exhibit more extensive collateral circulation. The collateral circulation primarily arises from the dilated bilateral subclavian arteries and their internal thoracic arteries, as well as branches such as the costocervical trunk, transverse cervical artery, thyrocervical trunk, suprascapular artery, subscapular artery, supreme intercostal artery, lateral thoracic artery, musculophrenic artery, superior epigastric artery, and anterior spinal artery. Occasionally, the subclavian artery becomes extremely dilated, resembling an aneurysm. The intercostal arteries primarily involved in forming collateral circulation are the 4th to 7th pairs. The common carotid artery participates in collateral circulation only in rare cases, such as when the subclavian artery is also stenotic or when the aortic coarctation is located proximal to the subclavian artery. In very few cases where the aortic coarctation is located in the mid-to-lower segment of the descending thoracic aorta or the abdominal aorta, the coarctation lesion covers a longer segment, the aorta above the lesion gradually narrows, and the collateral circulation is underdeveloped and atypical.
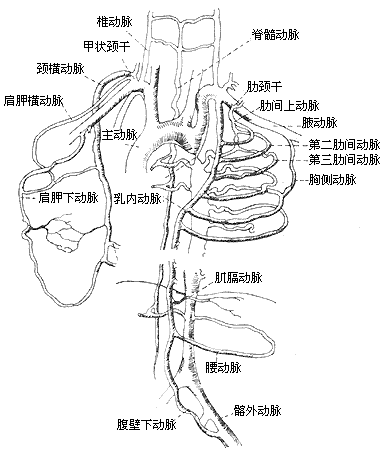
Figure 9 Collateral Circulation
The narrowing of the main artery increases blood flow resistance, leading to elevated blood pressure proximal to the narrowing and reduced blood supply and lower blood pressure distal to the narrowing. Gupta and Wiggers found that reducing the lumen of the main artery by 50% in experimental animals caused an increase in systolic blood pressure above the narrowing and a decrease below it, indicating that mechanical narrowing is the primary cause of hypertension. Scott and Bahnson first transplanted one kidney of an experimental dog to the neck and then created experimental narrowing of the main artery. Postoperative hypertension in the upper limbs could be alleviated by removing the kidney with reduced blood supply below the narrowing. Additionally, clinical cases of main artery narrowing often show elevated plasma renin levels, suggesting that the hypertension in these cases is related not only to mechanical factors but also to renal ischemia and the action of renin. In some cases of main artery narrowing, even after surgical removal of the narrowed segment and the elimination of the pressure gradient between the proximal and distal main artery, hypertension persists. This has led some to speculate that it may be related to dysfunction of the baroreceptors in the ascending main artery wall or adrenal abnormalities. The hypertension proximal to the narrowing and the formation of collateral circulation, along with associated congenital cardiovascular malformations, severely disrupt normal circulatory function and threaten the patient's life. Common fatal causes include congestive heart failure, bacterial endocarditis or endarteritis, main artery rupture, cerebrovascular accidents, and rupture of cerebral artery aneurysms such as those in the circle of Willis. According to Gross's statistics, 90% of preductal main artery narrowing cases die from heart failure within the first year of life. For postductal main artery narrowing cases, Abbott's 1928 autopsy data showed an average age of death at 32 years. Reifenstein's 1947 review of 104 autopsy cases revealed that 61% of patients died before the age of 40.
bubble_chart Clinical Manifestations
The clinical manifestations of aortic coarctation vary depending on the location of the coarctation segment, the degree of narrowing, the presence of other cardiovascular malformations, and the age group.
**Infancy and early childhood**: In cases of simple postductal aortic coarctation, although hypertension exists, clinical symptoms generally do not appear during infancy. However, in cases with other congenital cardiovascular malformations or preductal aortic coarctation, the most common clinical symptom is congestive heart failure. Approximately half of the cases begin to show symptoms such as rapid breathing, increased heart rate, sweating, feeding difficulties, hepatomegaly, and cardiomegaly within one month after birth when the ductus arteriosus closes. Left ventricular failure in infants and young children is often difficult to control with medical treatment. In severe cases of preductal aortic coarctation with a large and patent ductus arteriosus, due to the right-to-left shunt in the descending aorta, cyanosis may appear in the toes and sometimes the left hand, while the right hand and lips remain normal in color. In such cases, femoral pulses are normal, and no murmurs are heard in the ductus arteriosus region. However, because ventricular septal defects or atrial septal defects are often present, with a large left-to-right shunt within the heart chambers, cyanosis in the lower half of the body is uncommon. In critically ill cases with severely reduced left cardiac output, cyanosis may occur.
Although hypertension may occur in infants and young children with aortic coarctation, the degree of blood pressure elevation is not severe, with upper limb blood pressure typically exceeding lower limb blood pressure by more than 2.7 kPa (20 mmHg). Cardiomegaly is often present. Heart murmurs may be absent, or systolic murmurs and gallop rhythms may be heard along the left sternal border and the back corresponding to the coarctation segment. Weakened or absent femoral pulses are common. Although enlarged collateral circulation vessels may be visible on angiocardiograms, they are difficult to palpate. Chest X-rays show significant and progressively increasing cardiomegaly, with marked pulmonary vascular congestion. Electrocardiograms may reveal right ventricular hypertrophy within months after birth, and biventricular hypertrophy or left ventricular hypertrophy may appear after six months. Two-dimensional echocardiography can display the aortic coarctation segment. Aortic catheterization can assess the severity of coarctation based on the systolic pressure gradient between the proximal and distal ends of the coarctation segment. Aortography not only confirms the diagnosis and provides information on the location, length, and degree of narrowing of the coarctation segment but also reveals collateral circulation vessels, assesses the development of the ascending aorta and aortic arch, and checks for abnormalities in the distribution of aortic branches or complications such as aneurysms.
Childhood and adulthood: In cases of aortic coarctation without other congenital cardiovascular anomalies, most individuals do not exhibit clinical symptoms during childhood. The condition is often discovered during physical examinations, which reveal upper limb hypertension, weakened or absent femoral pulses, heart murmurs, or abnormalities on chest X-rays, prompting further investigation to confirm the diagnosis. In patients over 1 year old, approximately 5% experience symptoms such as headaches, shortness of breath after exertion, palpitations, fatigue, strong pulsations in the head and neck vessels, and epistaxis. A few cases may exhibit coldness in the lower limbs, weakness while walking, or even intermittent claudication due to reduced blood supply to the lower body. Rupture of intracranial aneurysms can lead to subarachnoid hemorrhage. Enlarged intercostal arteries compressing the anterior spinal artery may cause lower limb paralysis. Adults with the condition often present with hypertension, heart failure, and may die from complications such as bacterial endocarditis, endarteritis, or aortic rupture. Physical examination typically shows normal growth and development, with strong radial pulses but weakened or absent femoral pulses. Lower limb pulses appear delayed compared to upper limb pulses, and upper limb blood pressure is significantly higher than in the lower limbs. If the coarctation involves the left subclavian artery, blood pressure in the right arm will be higher than in the left. In cases with well-developed collateral circulation, pulsations of collateral vessels may be visible and palpable above the sternal notch and in the interscapular region. A systolic murmur is often heard along the left sternal border and may radiate to the back. Fundoscopic examination may reveal hypertensive changes in the retinal arteries. Chest X-ray findings become more pronounced with age. Children may show no abnormalities, but patients over 10 years old often exhibit cardiomegaly, particularly of the left ventricle. The aortic arch shadow may diminish, and a "3" sign may appear due to post-stenotic dilation of the descending aorta below the coarctation and dilation of the left subclavian artery. Notching of the inferior rib margins caused by enlarged, tortuous intercostal arteries is a characteristic X-ray feature of aortic coarctation. Rib notching is only seen in patients over 5 years old, most commonly affecting the 4th to 9th ribs bilaterally. However, if the subclavian artery is involved, rib notching may be absent on the affected side. Barium swallow studies may reveal an "E" sign, caused by the post-stenotic dilation of the descending aorta or enlarged right intercostal arteries compressing the left wall of the esophagus.
bubble_chart Auxiliary Examination
Aortic angiography can clearly determine the location and length of the narrowed segment, the degree of aortic lumen stenosis, the distribution of the ascending aorta and aortic arch branches and whether they are affected, as well as the collateral circulation. Sometimes, it may also reveal a patent ductus arteriosus. For typical cases of aortic coarctation, routine aortic angiography is not necessary. However, for cases with abnormal locations of the narrowed segment or long-segment aortic coarctation—such as those with murmurs audible in the lower back or rib notching limited to one side or at a lower position—the data provided by aortic angiography can aid in surgical planning.
Electrocardiogram (ECG): ECG changes depend on the severity of the coarctation and hypertension, as well as the duration of the condition. In childhood cases, ECG may show no abnormalities, while older patients often exhibit left ventricular hypertrophy and strain. If other cardiovascular lesions are present, ECG may show biventricular hypertrophy or right ventricular hypertrophy. For adult cases, if the ECG reveals myocardial damage or bundle branch block, careful consideration should be given to whether the patient can tolerate surgical treatment.
Cardiac catheterization: A catheter is inserted via the femoral artery and advanced into the descending aorta. If it passes through the narrowed segment, the aortic pressure proximal to the coarctation can be measured. The catheter is then slowly withdrawn while continuously recording aortic pressure. As the catheter passes through the narrowed segment, blood pressure immediately and abruptly drops. A significant pressure gradient between the proximal and distal ends of the coarctation not only confirms the diagnosis but also helps assess the severity of the coarctation. For patients with other cardiovascular lesions, cardiac catheterization and angiography can provide important diagnostic information. Two-dimensional echocardiography can also reveal aortic coarctation.
bubble_chart Treatment Measures
The treatment goal for coarctation of the aorta is to resect the narrowed segment and reconstruct the normal blood flow channel of the aorta, restoring normal blood pressure and circulatory function.
Cases of aortic coarctation accompanied by other congenital cardiovascular malformations may present with heart failure during infancy, leading to death. For those without other severe congenital cardiovascular malformations, complications such as aortic aneurysm, aortic rupture, bacterial endocarditis or endarteritis, as well as persistent long-term hypertension leading to cerebrovascular accidents, congestive heart failure, and coronary artery disease, may develop with age. Therefore, once diagnosed, surgical treatment should be considered for aortic coarctation cases, but the timing and method of surgery depend on the patient's age and cardiovascular condition. Infants with other severe congenital cardiovascular malformations and clinical heart failure have a mortality rate as high as 80% without surgical treatment. The surgical mortality rate for resection of the coarctation segment and aortic anastomosis was once as high as 56% in earlier years. With improvements in preoperative preparation, anesthesia, surgical techniques, and postoperative care, the mortality rate has now decreased to around 15%. Currently, it is recommended that severely ill infants be immediately administered intravenous prostaglandin E at 0.1 mg per kilogram per minute to delay the closure of the ductus arteriosus. Approximately 80% of infants show rapid improvement after administration, with femoral pulses appearing and metabolic acidosis due to inadequate lower body perfusion resolving. Surgery can be performed after 6–12 hours of sustained improvement. For cases unresponsive to medication, immediate surgery is advised. After resection and end-to-end anastomosis of the coarctation segment in infants, about 16–50% of cases develop re-coarctation within one year. Therefore, surgical methods such as using a subclavian artery flap or patch augmentation are preferred. For postductal aortic coarctation cases presenting with congestive heart failure in infancy, surgery can be delayed if heart failure resolves completely with medical treatment. In childhood, although most cases do not show obvious symptoms, surgery is still necessary to prevent the adverse effects of persistent hypertension on the cardiovascular system. Resection of the coarctation segment and end-to-end anastomosis of the proximal and distal aorta are generally recommended at 3–4 years of age. By this time, the aortic diameter is larger, secondary vascular changes due to hypertension are less pronounced, vessel elasticity is good, surgical procedures are more feasible, safety is higher, outcomes are better, and postoperative hypertension recurrence is rare. Surgery performed too early, when the aortic diameter is small, carries a high risk of re-coarctation due to limited growth potential of the anastomotic ring. Delayed surgery, on the other hand, may result in reduced vascular elasticity and increased fragility. Cases complicated by aortic or intercostal artery aneurysms present greater surgical challenges, higher mortality rates, and more severe cardiovascular damage from hypertension, adversely affecting treatment outcomes.
Main pulmonary artery coarctation segment resection and proximal-distal main pulmonary artery anastomosis: In 1945, Crafoord and Nylin, as well as Gross and Hufnagel, independently performed the resection of the coarctation segment of the main pulmonary artery and end-to-end anastomosis of the proximal and distal main pulmonary artery, achieving success in treating main pulmonary artery coarctation. This procedure is the most commonly used method for treating post-ductal main pulmonary artery coarctation. Preoperative detailed examinations of the heart and the functions of vital organs such as the liver and kidneys should be conducted, and the location and length of the coarctation segment should be accurately assessed. For cases where the coarctation segment is relatively long and the end-to-end anastomosis of the main pulmonary artery is expected to be technically challenging, vascular substitutes should be prepared preoperatively to facilitate vascular grafting. Due to the abundant collateral circulation, significant blood loss may occur during the incision of the chest wall; therefore, an adequate supply of banked blood should be prepared preoperatively. Intravenous infusion and transfusion pathways must be unobstructed, and a plastic infusion tube is typically inserted into the ankle vein before thoracotomy. To prevent ischemic spinal cord damage that may result from clamping the main pulmonary artery during surgery, systemic hypothermic anesthesia is recommended, with the body temperature lowered to 32–30°C. To avoid excessive elevation of upper-body blood pressure due to the interruption of main pulmonary artery blood flow, facilitate surgical manipulation, and reduce blood loss, the anesthesia team should prepare controlled hypotensive measures during the procedure. The patient is placed in the right lateral decubitus position, and a left posterolateral thoracotomy incision is made. The enlarged collateral vessels within the chest wall tissues must be individually clamped, divided, and ligated to minimize blood loss. A long incision through the fourth intercostal space or subperiosteal resection of the fifth rib via the rib bed is performed to access the thoracic cavity. If necessary, the posterior ends of 1–2 ribs above and below the incision may be excised to improve surgical exposure. The mediastinal pleura covering the left subclavian artery and the thoracic main pulmonary artery is longitudinally incised, and the left subclavian artery and the proximal and distal main pulmonary artery segments distant from the coarctation are freed and encircled with tape. The traction provided by the tape facilitates further dissection of the intercostal arteries, the coarctation segment of the main pulmonary artery, and the arterial duct or ligament. The intercostal arteries are important branches of the collateral circulation and should be preserved as much as possible during the dissection of the main pulmonary artery. Before incising the main pulmonary artery, sutures or gentle atraumatic vascular clamps may be temporarily applied to block the blood flow of the intercostal arteries, which are removed or loosened after the main pulmonary artery anastomosis is completed. If necessary, 1–2 pairs of intercostal arteries may be divided to facilitate the end-to-end anastomosis of the main pulmonary artery. When dissecting, dividing, and ligating the intercostal arteries, care should be taken to stay as far away from the main pulmonary artery wall as possible, as the intercostal arteries near the main pulmonary artery are more fragile and prone to rupture, leading to difficult-to-control bleeding. After ligating and dividing the arterial duct or ligament, the coarctation segment and the proximal and distal main pulmonary artery segments are fully mobilized, and atraumatic vascular clamps are then placed on the proximal and distal main pulmonary artery segments. The clamps should be positioned as far away from the coarctation segment as possible, ensuring that sufficient length of the main pulmonary artery remains after resection of the coarctation segment to facilitate anastomosis. The proximal main pulmonary artery clamp should include the inferior wall of the main pulmonary artery arch, while the distal main pulmonary artery clamp may also include the intercostal vessels. After clamping the main pulmonary artery, if the systolic blood pressure in the upper body rises above 20.0 kPa (150 mmHg), drugs such as arfonad or sodium nitroprusside should be administered to control the blood pressure. The coarctation lesion must be completely resected to avoid residual stenosis that could result in an overly small anastomotic orifice, impairing blood flow. For coarctation segments resected within 2 cm in length, end-to-end anastomosis of the main pulmonary artery can generally be performed (Figure 1). To increase the internal diameter of the anastomosis, the proximal and distal ends of the coarctation segment may be obliquely transected to enlarge the vascular diameter. However, if the resected segment exceeds 2 cm, performing an end-to-end anastomosis may create excessive tension, and it is advisable to implant a segment of artificial vascular graft or homologous main pulmonary artery between the cut ends of the proximal and distal main pulmonary artery segments.
Figure 1 Resection of the narrowed segment and end-to-end anastomosis
(1) Free the proximal and distal vessels of the narrowed segment and loop them with gauze tapes; (2) Define the resection range of the narrowed segment; (3) Anastomose the posterior wall; (4) Completion of the posterior wall anastomosis; (5) Completion of the anterior wall anastomosis
When performing an end-to-end anastomosis of the main stirred pulse, the assistant should bring the vascular clamps on the proximal and distal segments of the main stirred pulse close together and maintain stability. First, suture and ligate the side walls of both ends with a penetrating stitch, then use non-traumatic synthetic sutures and fine needles to continuously suture the full thickness of the posterior wall of the anastomosis. After tying the sutures on the side walls, continuously suture the full thickness of the anterior wall. When using non-absorbable materials such as silk for the anastomosis, the anterior wall of the anastomosis should be sutured intermittently to avoid hindering the anastomosis from enlarging as the body grows postoperatively.
After completing the anastomosis, first release the vascular clamp on the distal main stirred pulse to allow blood to flow upward into the anastomosis site, expelling any trapped air in the main stirred pulse lumen. If there is fistula disease bleeding at the anastomosis site, additional sutures may be needed. Then, discontinue the hypotensive drugs, increase the blood transfusion rate, administer peripheral vasoconstrictors, and gradually release the proximal main stirred pulse vascular clamp slowly to prevent a sudden, drastic drop in blood pressure, which could lead to declamping shock and ventricular fibrillation. After rechecking the anastomosis, suture the mediastinal pleura, place a thoracic drainage tube, and close the chest wall incision layer by layer. Finally, initiate rewarming.
If the narrowed segment is too long to allow end-to-end anastomosis after resection, or if the tension during end-to-end anastomosis is too great, or if the distal main stirred pulse or intercostal stirred pulse also has stirred pulse tumor diseases requiring simultaneous resection, a segment of vascular graft should be used for main stirred pulse transplantation. In 1951, Gross reported the clinical experience of using homologous main stirred pulse transplantation to treat 19 cases of main stirred pulse narrowing. With the continuous advancement of artificial blood vessels, polyester fiber or Gortex grafts are now commonly used in clinical practice. For main stirred pulse transplantation, select an artificial blood vessel of appropriate diameter and length. The suturing methods for the proximal and distal anastomoses are the same as those for end-to-end anastomosis of the main stirred pulse (Figure 2).
Figure 2 Resection of the narrowed segment and artificial blood vessel transplantation
For older patients with sclerotic changes in the main stirred pulse wall, the narrowed segment and the proximal and distal main stirred pulse can be incised. After removing the thickened inner membrane and medial layer of the narrowed area, use a wide water calptrop base peel-shaped polymer patch to enlarge the main stirred pulse (Figure 3).
(1) Incision of the narrowed segment
(2) Removal of the thickened inner membrane and medial layer tissue
(3) Ligate the stirred pulse ligament and patch the narrowed segment for enlargement
(4) The narrowed segment has been enlarged
Figure 3 Enlargement and patch repair of the main stirred pulse narrowed segment
Application of subclavian artery flap for primary artery plasty: The surgical mortality rate for resection of the coarctation segment in infants and young children with primary artery coarctation is high. Moreover, due to the small diameter of the primary artery—only 50% of that in adults—the annular scar tissue formed after end-to-end anastomosis cannot expand with physical growth, leading to a high incidence of postoperative primary artery restenosis. In 1966, Waldhausen and Nahrwold proposed using the subclavian artery flap to repair and enlarge the primary artery coarctation in infants and young children, significantly reducing the incidence of postoperative primary artery restenosis. Preoperative preparation, anesthesia methods, and surgical incisions are the same as those for resection of the primary artery coarctation segment. After entering the thoracic cavity, the primary artery and left subclavian artery are dissected, and the arterial duct or arterial ligament is ligated and divided. For larger arterial ducts, division and suturing are required. The left subclavian artery and vertebral artery are ligated at the apex of the thoracic cavity. Ligation of the vertebral artery prevents postoperative subclavian artery steal syndrome. A vascular clamp is placed on the proximal segment of the primary artery between the left subclavian artery and the left common carotid artery, and another clamp is placed on the descending primary artery below the coarctation segment. The left subclavian artery is divided at the apex of the thoracic cavity, and then the entire length of the left subclavian artery is longitudinally incised. The lower edge of the incision extends to the primary artery coarctation segment and approximately 1 cm of the descending primary artery below the coarctation segment. The thickened intimal membrane and medial tissue forming the septal membrane within the coarctation segment of the primary artery are excised. The left subclavian artery flap is then flipped downward. First, a suture is used to ligate the flap to the lower edge of the primary artery incision, followed by continuous or interrupted suturing of the anterior and posterior edges of the flap to the corresponding edges of the primary artery incision. Since the suture line is U-shaped, the future growth capacity of the anterior and posterior walls of the primary artery remains unaffected, preventing postoperative restenosis (Figure 4).
Figure 4 Application of subclavian stirred pulse flap for main stirred pulse plasty
The above surgical methods, especially resection of the narrowed segment with end-to-end anastomosis of the main stirred pulse and the use of a subclavian stirred pulse flap for main stirred pulse plasty, are the most commonly used surgical treatments for main stirred pulse coarctation. Currently, the surgical mortality rate has been reduced to 1-2%.
For a very small number of cases with special ranges and locations of the coarctation lesion, the following surgical treatments can also be used: wedge resection of the main stirred pulse coarctation lesion, also known as the Walker procedure. This involves wedge resection of part of the main stirred pulse wall followed by transverse approximation and suturing of the main stirred pulse incision (Figure 5). This procedure is only suitable for cases where the coarctation lesion is limited to the lateral wall of the main stirred pulse, the lesion length is extremely short and involves less than 50% of the main stirred pulse circumference, and the main stirred pulse diameters at both ends of the coarctation segment are relatively large with normal vascular walls.
Figure 5 Walker procedure
Artificial vascular bypass graft: For cases where the coarctation lesion is located in the aortic arch proximal to the left subclavian stirred pulse or involves a long segment of descending aortic coarctation, a segment of artificial vessel can be grafted between the ascending aorta and the thoracic descending aorta for the former, or between the thoracic aorta and the distal descending aorta or abdominal aorta beyond the coarctation segment for the latter (Figure 6).
Figure 6 Artificial vascular bypass graft
Subclavian stirred pulse-descending aorta anastomosis: In the 1950s, Blalock and Clagett ligated and transected the left subclavian stirred pulse, turned its proximal segment downward, and performed an end-to-side anastomosis with the thoracic aorta distal to the stenosis, or simultaneously resected the coarctation segment and performed an end-to-end anastomosis between the proximal subclavian stirred pulse and the thoracic aorta (Figure 7). However, since the diameter of the left subclavian stirred pulse is smaller than that of the aorta in the vast majority of cases, and the subclavian stirred pulse is prone to twisting at its root after being turned downward, affecting blood flow, the efficacy is unsatisfactory, and this method is rarely used.
Figure 7 Subclavian stirred pulse-descending aorta anastomosis
Percutaneous balloon catheter aortic dilation plasty has not been used clinically for a long time. It is more suitable for infant cases with very short coarctation segments and has better efficacy for cases with residual stenosis or restenosis after surgery. However, its efficacy as a first-line treatment is still not entirely satisfactory, and there may be residual pressure gradients after dilation. The long-term efficacy still requires further observation.
Postoperative complications: Possible complications after resection of the coarctation segment with end-to-end anastomosis of the aorta or subclavian stirred pulse flap aortic plasty for aortic coarctation cases include:
(1) Postoperative hypertension: After proper correction of the aortic coarctation lesion, most cases may still exhibit elevated systolic or diastolic blood pressure in the early postoperative period, with varying durations. About 10% of cases may experience abdominal discomfort, abdominal distension and fullness, or abdominal pain in the first week after surgery, along with fever, leukocytosis, abdominal tenderness, and weakened bowel movements. In 1957, Sealy observed that abdominal pain mostly occurs within 48 hours after surgery. The cause of hypertension in cases with delayed onset, primarily characterized by elevated diastolic blood pressure, may be due to abnormal regulation of vascular wall baroreceptors, increased secretion of epinephrine and norepinephrine, or elevated plasma renin-angiotensin levels. To prevent hypertension, sodium nitroprusside can be administered intravenously within 24 hours postoperatively to maintain systolic blood pressure at around 14.7 kPa (110 mmHg), followed by oral antihypertensive medications after 24 hours.
In cases where the narrowing of the lesion is not completely corrected during surgery or restenosis occurs postoperatively, hypertension persists, and there remains a blood pressure difference of more than 14.7 kPa (110 mmHg) between the upper and lower limbs. Clinical examination may reveal that the femoral pulse is weaker than the brachial or radial pulse and appears delayed, with lower blood pressure in the lower limbs compared to the upper limbs. In cases with a significant pressure difference between the upper and lower limbs, aortography can show a narrowed aortic lumen at the original surgical site.
The narrowing lesion persists without resolution, and the main causes of persistent stenosis are improper surgical techniques, such as insufficient resection length of the narrowed segment, remaining small lumen of the main artery, inadequate diameter after end-to-end anastomosis, inappropriate diameter or length of the graft used in artificial vascular transplantation, incomplete removal of the narrowed segment membrane tissue during aortic plasty, improper trimming of the subclavian artery flap or synthetic patch; or twisting of the applied artificial graft or subclavian artery during bypass grafting or shunt surgery. A common cause of postoperative restenosis is the failure of the anastomotic site to grow proportionally with the body's development after end-to-end anastomosis of the main artery, leading to restenosis. Performing resection of the narrowed segment and end-to-end anastomosis of the main artery during infancy, especially with continuous suturing around the entire circumference of the main artery and using non-absorbable sutures such as silk threads, results in a higher complication rate. Restenosis rarely occurs after aortic plasty. Tissue trauma to the aortic wall caused by vascular clamps during surgery, or postoperative hyperplasia of abnormal mesodermal tissue remaining in the aortic wall, can also lead to intimal and medial thickening, resulting in restenosis.
Long-term follow-up of patients with aortic coarctation shows that the incidence of hypertension is 4–5 times higher than in the general population. Patients who undergo surgery at the age of 20 or older have an even higher long-term incidence of postoperative hypertension.
(2) Spinal Cord Ischemic Injury During surgery for aortic coarctation, clamping the proximal and distal segments of the narrowed aorta, and sometimes the left subclavian artery, reduces spinal cord blood supply, leading to ischemic injury and varying degrees of postoperative lower limb paralysis. However, most patients have abundant collateral circulation between the proximal and distal segments of the narrowed aorta, making spinal cord ischemic injury rare, with a complication rate of about 0.5%. In infants with aortic coarctation, if the narrowed segment is located proximal to the left subclavian artery and accompanied by stenosis at the root of the left subclavian artery, or in cases of preductal coarctation where the descending aortic blood supply comes from the ductus arteriosus, abnormal vascular anatomy supplying the spinal cord, or very mild coarctation, collateral circulation may be underdeveloped. Excessive intraoperative resection of intercostal arteries, significant blood loss, hypotension, or prolonged aortic clamping time all increase the risk of postoperative spinal cord ischemic injury.
The use of hypothermic anesthesia, preservation of intercostal arteries as much as possible, shortening aortic clamping time, and preventing excessive intraoperative blood loss leading to hypotension can all help avoid postoperative spinal cord ischemic injury. For patients with underdeveloped collateral circulation, left heart-femoral artery bypass or temporary placement of a blood shunt between the proximal and distal aortic segments can maintain blood supply to the lower body and spinal cord.
(3) Chylothorax About 5% of patients with aortic coarctation develop postoperative chylothorax due to intraoperative damage to the thoracic duct or its branches. Early postoperative chylothorax with minimal chyle leakage may resolve after drainage via a chest tube. However, if chyle leakage is substantial, persists for more than a week, and affects nutritional status, reoperation is required to locate and suture the thoracic duct or its branches. If the leakage site cannot be identified, the thoracic duct should be doubly ligated behind the esophagus. Some cases present with delayed chylothorax one week postoperatively, so a chest X-ray should be reviewed one week after surgery. If pleural effusion is found, thoracentesis should be performed immediately to determine the nature of the fluid. Once chylothorax is confirmed, repeated thoracentesis every 3–4 days can remove the chyle, and most cases can be cured. If multiple thoracenteses fail, reoperation to suture or ligate the thoracic duct is necessary.
(4) Aneurysm or pseudoaneurysm is a serious complication after aortic coarctation repair. Pseudoaneurysms occurring in the early postoperative period are mostly caused by improper suturing techniques, fistula disease at the suture site, rupture, or bacterial infection at the anastomosis. After aortic reconstruction using a Dacron patch, due to the rigidity of the patch, the normal aortic wall is subjected to long-term pulsation and tension from blood flow, making it prone to aneurysm formation. In a few cases, aneurysm develops gradually due to postoperative dissection of the proximal and distal aortic walls.
The surgical mortality rate for postductal coarctation of the aorta is generally less than 3%. Common causes of death include heart failure, inadequate pulmonary circulation, improper technical procedures, and massive hemorrhage due to rupture of blood vessels or aortic aneurysms. Infants under 1 year of age have a higher surgical mortality rate due to the severity of their condition compared to patients over 1 year old. The presence of other congenital cardiovascular anomalies also increases surgical mortality. For cases accompanied by ventricular septal defects, the surgical mortality rate is 20–30%, while for those with other severe cardiovascular anomalies, it can be as high as 50–70%.
The 15-year postoperative survival rate for simple postductal coarctation of the aorta exceeds 90%, but it drops to 80% for cases with ventricular septal defects and further declines to 40% for those with other severe cardiovascular anomalies. Patients over 20 years of age at the time of surgery also experience reduced long-term survival rates. Common long-term causes of death include myocardial infarction, aortic valve disease, rupture of aortic aneurysms, and hypertension or heart failure caused by residual or recurrent stenosis. Therefore, surgical treatment should be performed for all cases of simple postductal coarctation of the aorta once diagnosed. Surgery should be performed as early as possible for patients over 3–4 years of age. Immediate surgery is recommended for cases with upper limb blood pressure exceeding 20 kPa (150 mmHg) or uncontrolled heart failure despite medical treatment. However, caution should be exercised in cases with other severe congenital cardiovascular anomalies, pulmonary insufficiency, congestive heart failure, myocardial damage or conduction block on electrocardiogram, extensive atherosclerotic or calcified lesions in the aortic wall, or insufficient coronary blood supply.
Aortic coarctation is often associated with other congenital cardiovascular anomalies. The most common ones include patent ductus arteriosus and bicuspid aortic valve. Additionally, it may be accompanied by aortic valve stenosis, ventricular septal defect, hypoplastic ascending aorta, and endocardial fibroelastosis. Approximately half of Turner syndrome (also known as X syndrome) cases are associated with aortic coarctation. Turner syndrome is a congenital ovarian dysgenesis caused by abnormal sex chromosomes. The main clinical manifestations include short stature, delayed physical growth and sexual development, loose neck skin that gradually forms a webbed neck, low posterior hairline, and cubitus valgus.




