| disease | Atrial Septal Defect |
Atrial septal defect is a relatively common congenital heart disease. According to clinical data from Beijing and Shanghai, atrial septal defects account for 24.6% and 21.7% of various congenital heart diseases, respectively. This condition is more prevalent in females, with a female-to-male ratio ranging from 2:1 to 4:1. Atrial septal defect is caused by the underdevelopment of tissues related to the formation of the atrial septum during the embryonic stage.
bubble_chart Pathogenesis
During the fetal period, the lungs do not perform respiratory functions and remain in an atelectatic state. The pulmonary circulation has high resistance, resulting in minimal blood flow. Therefore, blood returning to the right atrium must pass through the interatrial septum into the left atrium to meet the unique circulatory physiological demands of the fetal stage. To accommodate this, the interatrial septum maintains an interatrial foramen throughout its development, which closes after birth. Around the end of the first month of embryogenesis, the septum primum grows from the dorsal upper part of the primitive atrial wall along the midline. Simultaneously, endocardial cushions develop from the dorsal and ventral sides of the atrioventricular junction. During development, these two endocardial cushions gradually enlarge and fuse. Their upper portions connect with the interatrial septum, while their lower portions form the membranous part of the interventricular septum, linking with the muscular part of the interventricular septum. The endocardial cushion tissues on both sides of the atrioventricular junction develop into the atrioventricular valve structures—the septal leaflet of the tricuspid valve on the right and the anterior leaflet of the mitral valve on the left. The septum primum, shaped like a horseshoe, grows toward the endocardial cushions. Its anterior and posterior portions fuse with the corresponding endocardial cushions, while the central part retains a crescent-shaped interatrial foramen, known as the foramen primum, through which blood flows from the right atrium to the left atrium. As the central part of the septum primum fuses with the endocardial cushions and the foramen primum begins to close, the upper tissue of the septum primum undergoes resorption, forming another interatrial foramen called the foramen secundum, maintaining blood flow between the atria. Subsequently, another septum, the septum secundum, grows from the atrial wall on the right side of the septum primum. The septum secundum is also horseshoe-shaped. Its anterior-inferior end fuses with the ventral endocardial cushion and divides into two parts: one extends posteriorly along the base of the septum primum to connect with the later-developed end of the septum secundum, forming the lower margin of the foramen ovale. The other grows between the coronary sinus and the inferior vena cava, contributing to the formation of the valve of the inferior vena cava. The oval-shaped opening in the central part of the septum secundum is called the foramen ovale. The left side of the foramen ovale is covered by the septum primum (the valve of the foramen ovale), forming a shallow depression known as the fossa ovalis. By the eighth week of embryogenesis, the development of the interatrial septum is complete. The septum primum and septum secundum fuse, leaving only a blood flow passage between the atria at the upper part of the fossa ovalis and the valve of the foramen ovale. However, due to the valve-like function of the foramen ovale, blood can only flow from the right atrium through the fossa ovalis and foramen secundum into the left atrium. Complete fusion of the foramen ovale and its valve occurs after birth (Figure 6). However, according to pathological anatomy data, the foramen ovale remains patent in about 20–30% of newborns. After birth, as the lungs expand and pulmonary vascular resistance decreases, pulmonary blood flow increases, raising left atrial pressure above right atrial pressure. This causes the valve of the foramen ovale to press tightly against the fossa ovalis. Thus, even if the foramen ovale remains anatomically patent, no significant interatrial blood shunting occurs under normal physiological conditions. However, in pathological conditions such as pulmonary stenosis or right ventricular outflow tract obstruction, elevated right atrial pressure can lead to right-to-left shunting, allowing blood to pass through the patent foramen ovale into the left atrium (Figure 6).
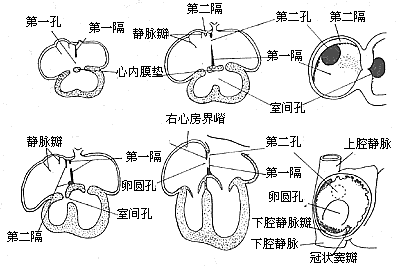
**Figure 6 Embryology of the Interatrial Septum**
From the developmental process of the interatrial septum, it can be observed that: - **Secundum-type atrial septal defects** result from underdevelopment of the septum secundum or the valve of the foramen ovale. - **Primum-type atrial septal defects** occur due to incomplete fusion of the endocardial cushions with the septum primum. - **Atrioventricular canal defects** arise from severe underdevelopment of the endocardial cushions, leading not only to a patent foramen primum but also to clefts in the septal leaflets of the mitral and tricuspid valves, and in severe cases, membranous ventricular septal defects. - **Common atrium** results from the absence or failure of development of the interatrial septum.
Type II atrial septal defect: In the vast majority of cases, the defect is solitary, although occasionally there may be two or more small holes. Based on the location of the defect, it can be classified into the following types (Figure 7).

Figure 7 Types of Atrial Septal Defects
1. Fossa Ovalis Defect This is the most common type, accounting for approximately 70% of atrial septal defect cases. The defect is located in the central portion of the atrial septum, corresponding to the embryonic fossa ovalis. It is generally oval or circular in shape, with a relatively large area, and the diameter mostly ranges from 2 to 4 cm or larger. Most cases present as a single large opening, but it may also be divided into multiple small holes by irregular strands of residual septum primum tissue (the valve of the foramen ovale), resembling a sieve. In most cases, the edges of the defect are intact. The coronary sinus opening is located anteriorly and inferiorly to the defect, and there is still a considerable amount of atrial septal tissue between the inferior edge of the defect and the atrioventricular valves. The defect is relatively far from the atrioventricular node, making it easier to avoid injury to the conduction tissue during suturing. In some cases, the defect is large, with very little or no atrial septal tissue at the posterior edge, and the opening of the right pulmonary vein into the defect area may be mistaken for partial anomalous pulmonary venous return.
2. Superior Vena Cava Type Defect or High Defect Also known as a sinus venosus defect, this accounts for approximately 5–10% of atrial septal defects. The defect is located at the junction of the superior vena cava and the right atrium. The inferior edge of the defect consists of atrial septal tissue. The defect area is generally small, rarely exceeding 2 cm. The superior edge of the defect is the superior vena cava, which straddles the left and right atria. High atrial septal defects are often accompanied by anomalous drainage of the right superior pulmonary vein into the right atrium or superior vena cava.3. Inferior Vena Cava Type Defect or Low Defect Also referred to as a posterior atrial septal defect, this accounts for approximately 20% of atrial septal defects. The defect is located in the to be decocted later portion of the atrial septum. The inferior edge of the defect is close to the entrance of the inferior vena cava, and there may still be a small amount of fossa ovalis tissue between the inferior edge and the inferior vena cava, or the atrial septal tissue may be entirely absent. There is no clear boundary between the inferior edge of the defect and the entrance of the inferior vena cava, and the valve of the inferior vena cava may be mistaken for the atrial septal tissue at the inferior edge. Care must be taken during surgery to avoid suturing in a way that diverts all inferior vena cava blood flow into the left atrium. The right pulmonary stirred pulse opening is located in the defect area and may also be accompanied by anomalous drainage of the right pulmonary vein into the right atrium or inferior vena cava.
Approximately 12% of secundum-type atrial septal defect cases may be associated with other congenital cardiovascular malformations or heart diseases, such as pulmonary stirred pulse valve stenosis, anomalous pulmonary venous return, ventricular septal defect, patent stirred pulse ductus arteriosus, mitral stenosis (Lutembacher syndrome), or persistent left superior vena cava.
The hemodynamic changes of a secundum atrial septal defect involve a blood shunt at the atrial level. Under normal circumstances, because the left ventricular muscle is thicker than the right ventricle, the blood flow resistance in the left heart and systemic circulation is higher than that in the right heart and pulmonary circulation. The average pressure in the left atrium is approximately 1.07–1.33 kPa (8–10 mmHg), while the normal average pressure in the right atrium is below 0.533–0.677 kPa (4–5 mmHg). Therefore, the direction of blood flow through the atrial septal defect is generally left-to-right, and clinically, cyanosis does not occur. The magnitude of the left-to-right shunt depends on the size of the defect, the compliance of the left and right ventricles, and the pressure gradient between the left and right atria. During infancy, the muscle thickness and compliance of the left and right ventricles, as well as the vascular resistance of the systemic and pulmonary circulations, are relatively similar, so the blood flow through the atrial septal defect is minimal. As age increases, pulmonary vascular resistance decreases, right ventricular pressure drops, and right ventricular myocardial compliance improves, leading to an increase in left-to-right shunt volume and pulmonary blood flow. The right atrium, right ventricle, and pulmonary artery gradually enlarge. As the right atrium expands, the size of the defect may also increase correspondingly, further increasing the shunt volume and making clinical symptoms more apparent. Right heart catheterization often reveals that pulmonary blood flow increases to 2–4 times the systemic blood flow. However, the left ventricular output can still maintain normal systemic circulation and blood pressure, except during strenuous exercise when the left ventricular output may fail to increase adequately. Although pulmonary blood flow increases significantly, due to the high compliance of the pulmonary vascular bed, pulmonary artery pressure does not rise in the early years. During childhood, among patients with atrial septal defects, only about 5% exhibit pulmonary hypertension with pulmonary artery pressure exceeding 6.67 kPa (50 mmHg) due to increased pulmonary vascular resistance. However, in patients over 40 years old, the incidence of pulmonary hypertension can reach 50%. Pathological changes in the pulmonary arterioles, such as medial hypertrophy and intimal hyperplasia caused by increased pulmonary blood flow, also become more common with age. In patients over 20 years old, pulmonary vascular obstructive disease, increased pulmonary vascular resistance, and pulmonary hypertension become significantly more prevalent. As pulmonary artery, right ventricular, and right atrial pressures gradually rise, the left-to-right shunt through the atrial septal defect decreases. If right atrial pressure exceeds left atrial pressure, a reverse shunt occurs, with some right atrial blood flowing into the left atrium through the defect. Once right-to-left shunting develops, cyanosis appears clinically. Pulmonary hypertension predisposes patients to respiratory infections and leads to hypertrophy and dilation of the right ventricle and atrium, eventually resulting in right heart failure and various atrial arrhythmias. In advanced cases of atrial septal defects with right-to-left shunting, surgical closure of the defect often exacerbates right heart failure, making such patients unsuitable for surgical intervention. Untreated patients with atrial septal defects have an average lifespan of around 50 years, with the primary cause of death being progressively worsening congestive heart failure.In cases of atrial septal defect combined with anomalous pulmonary venous drainage into the right atrium or atrial septal defect with mitral stenosis, the left-to-right shunt is greater. Conversely, in atrial septal defect with pulmonary stirred pulse or right ventricular outflow tract stenosis, depending on the degree of right atrial pressure elevation, the left-to-right shunt is smaller or manifests as a right-to-left shunt.
bubble_chart Clinical Manifestations
The secundum atrial septal defect typically has a small left-to-right shunt in the early stages. Most cases do not present significant clinical symptoms during childhood, and the condition is often identified during physical examinations due to the detection of a heart murmur, prompting further investigation. Clinical symptoms usually begin to manifest in adolescence when the left-to-right shunt increases, with the most common symptoms being fatigue, dyspnea on exertion, and palpitation. Patients with larger shunts and elevated pulmonary circulation pressure are prone to recurrent respiratory infections and pneumonia. Cases with partial anomalous pulmonary venous return and extremely large left-to-right shunts may present with heart failure in infancy, requiring early surgical intervention. The incidence of heart failure symptoms increases in patients over 30 years old. Cases complicated by pulmonary hypertension leading to heart failure, or those with severe pulmonary stenosis or right ventricular outflow tract obstruction, may develop a right-to-left shunt, clinically presenting as cyanosis.
**Physical Examination:** Most patients exhibit normal growth, development, and skin color, though some may appear relatively thin. Right ventricular enlargement may cause bulging of the left anterior chest wall. A heaving cardiac impulse can be palpated at the lower left sternal border. A systolic ejection murmur, caused by a large volume of blood flowing through the pulmonary valve into the dilated pulmonary artery, can be heard at the second or third left intercostal space. The pulmonary component of the second heart sound (P2) is accentuated and exhibits fixed splitting. Some cases may also have a palpable systolic thrill in the same area. A diastolic rumble may be heard at the tricuspid area due to rapid blood flow across the tricuspid valve. With the development of pulmonary hypertension, the systolic murmur at the pulmonary valve area diminishes, while the P2 becomes more pronounced. Cases with pulmonary valve insufficiency may exhibit a diastolic murmur at the second or third left intercostal space. Severe right ventricular dilation leading to relative tricuspid insufficiency may produce a systolic murmur at the tricuspid area. In cases with increased pulmonary vascular resistance, significantly reduced left-to-right shunting, or reversed shunting, heart murmurs may be less noticeable, and cyanosis may appear. **Advanced-stage** cases may present with signs of chronic congestive heart failure, such as jugular venous distension, edema, and hepatomegaly.
bubble_chart Auxiliary Examination
Chest X-ray examination: In cases with a large left-to-right shunt, chest X-ray examination reveals cardiac enlargement, particularly marked enlargement of the right atrium and right ventricle. The pulmonary artery trunk is prominently protruded, with enlarged blood vessels in both hilar regions and enhanced pulsations. Under fluoroscopy, hilar dance may sometimes be observed, along with thickened vascular markings in the lung fields. The shadow of the aortic arch is diminished. In patients with chronic congestive heart failure, due to compression of the trachea by extremely dilated small pulmonary vessels, X-ray signs such as interstitial pulmonary edema or atelectasis may be evident.
Electrocardiogram (ECG) examination: Typical cases often show right ventricular hypertrophy, incomplete or complete right bundle branch block, and right axis deviation. The P wave may be elevated or enlarged, and the P-R interval prolonged. The frontal plane vectorcardiogram shows a clockwise rotation of the QRS loop. In patients over 30 years old, supraventricular arrhythmias become increasingly common, initially manifesting as paroxysmal atrial fibrillation and later persisting. Among adult cases of atrial septal defect, approximately 20% present with atrial fibrillation.
Echocardiography: Echocardiography reveals an increased diameter of the right ventricle. During systole, the muscular portion of the interventricular septum on the left ventricular side moves forward in the same direction as the posterior wall of the left ventricle, contrary to the normal antagonistic motion—this is termed paradoxical septal motion. Two-dimensional echocardiography can directly visualize the location and size of the atrial septal defect.
Cardiac catheterization: Right heart catheterization is a reliable method for diagnosing atrial septal defects. Oxygen saturation in the right atrium, right ventricle, and pulmonary artery is higher than the average oxygen saturation in the systemic veins by 1.9 volume% or more, indicating a left-to-right shunt at the atrial level. Additionally, after entering the right atrium, the catheter may pass through the atrial septal defect into the left atrium. The range of the catheter's movement above and below the defect area can also help estimate the size of the defect. Catheters inserted via the great saphenous vein have a higher chance of passing through the atrial septal defect into the left atrium. The diagnosis of an atrial septal defect can be confirmed based on the abnormal catheter pathway and blood oxygen measurements. Cardiac catheterization can also measure pressures in various cardiac chambers, the pulmonary artery, and pulmonary capillaries. Notably, in cases of secundum-type atrial septal defects, due to increased pulmonary blood flow—especially in cases with a large left-to-right shunt—a systolic pressure gradient of 2.7 kPa (20 mmHg) may be observed between the right ventricle and the pulmonary artery, which should not be misdiagnosed as coexisting pulmonary valve stenosis. Data obtained from cardiac catheterization can be used to calculate cardiac output, systemic blood flow, pulmonary blood flow, left-to-right shunt volume, and pulmonary vascular resistance. These parameters are valuable for assessing defect size, detecting associated cardiovascular anomalies, and determining treatment strategies.
Angiography: After the catheter enters the left atrium, contrast injection and televised imaging can reveal the location and size of the atrial septal defect. Left ventricular angiography can determine whether mitral regurgitation is present. Contrast injection into the right pulmonary artery or right superior pulmonary vein helps confirm whether the right superior pulmonary vein anomalously drains into the superior vena cava or right atrium. Contrast injection into the pulmonary artery can demonstrate anomalous pulmonary venous drainage into the right atrium or inferior vena cava. Selective indicator-dilution curve measurements are highly valuable for confirming the diagnosis, assessing defect size, and estimating shunt volume. Moreover, in cases with small shunt volumes, indicator-dilution curve measurements are more sensitive than blood oxygen saturation measurements. Using hydrogen as an indicator inhaled via the respiratory tract, a platinum electrode-tipped catheter can record the hydrogen dilution curve in the right heart chambers. This method is highly sensitive, detecting the indicator's premature arrival curve (within less than 4 seconds) starting from the right atrium.
In recent years, advancements in echocardiography have progressed rapidly, enabling definitive diagnosis of secundum-type atrial septal defects. As a result, typical cases in children and adolescents without complications no longer routinely require invasive procedures such as right heart catheterization, angiography, or indicator-dilution curve measurements.
Based on clinical symptoms, signs, chest X-rays, electrocardiograms, and echocardiography, a diagnosis of secundum atrial septal defect can generally be made. In a few cases with atypical symptoms and signs, right heart catheterization may be performed when necessary. The diagnosis is further confirmed if the oxygen content in the right atrium is found to be more than 1.9% higher than that in the vena cava, and if the catheter can pass through the defect into the left atrium.
bubble_chart Treatment Measures
Some smaller cases of secundum atrial septal defects may close spontaneously within the first year after birth, but the likelihood of spontaneous closure is extremely small after two years. Surgical treatment should be considered for simple secundum atrial septal defects or secundum atrial septal defects with partial anomalous pulmonary venous return, where the ratio of pulmonary to systemic blood flow exceeds 1.5:1. The optimal age for surgery is 4–5 years, and early surgical intervention can prevent an increase in pulmonary vascular resistance and the onset of right heart failure. Infants presenting with congestive heart failure that is not controlled by medical management require early surgery.
A significant increase in pulmonary vascular resistance, reaching more than 6 Wood units at rest without reduction or further elevation after exercise, is a contraindication for surgery. Cases with clinical cyanosis, reversed shunting at the atrial level, and further reduction in arterial oxygen saturation after exercise are also contraindicated.
Surgical History: The development of extracorporeal circulation technology has provided an effective, reliable, and safe method for the surgical treatment of secundum atrial septal defects. A large number of cases have been treated surgically with good outcomes.
Before the advent of open-heart surgery, Murray in 1948 used sutures or broad fascia to pass through the atrial wall along the plane of the atrial septum, tightening the sutures to reduce the size of the defect, though the results were unsatisfactory. In 1954, Sondergaard used thick silk thread to pass through the anterior edge of the defect from outside the atrial wall and then through the interatrial groove, tying the suture to reduce or close the defect. Gross reported in 1952 the clamping of part of the right atrial wall and attaching a rubber "atrial well" to the atrial incision, allowing direct suturing of the defect without blood spillage. Bailey, Santy, and Cohn also developed other closed techniques for treating atrial septal defects. In 1953, Lewis, Taufic, and Swan independently used surface hypothermia anesthesia with caval occlusion to suture the defect under direct vision through a right atrial incision. In the same year, Gibbon employed a heart-lung machine for extracorporeal circulation to suture or patch the defect, achieving excellent results and ushering in a new era of open-heart surgery for various cardiac conditions.
Open-heart suturing of atrial septal defects provides a bloodless field, clear visualization of cardiac anatomy, and precise surgical correction, making it highly satisfactory and replacing closed techniques. In the early years of open-heart surgery, many secundum atrial septal defects were repaired under hypothermia with circulatory arrest. Hypothermic open-heart surgery does not require complex equipment like heart-lung machines but is limited to a safe intracardiac operating time of only 6–8 minutes, making it suitable only for fossa ovalis defects. Larger defects, superior or inferior vena cava-type defects requiring patch repair, or cases with concomitant cardiac anomalies requiring simultaneous correction exceed the safe time limit of hypothermia. Additionally, hypothermia can cause severe arrhythmias and cerebral hypoxia. With advancements in extracorporeal circulation technology, clinical practice has proven that open-heart surgery under extracorporeal circulation provides ample time for intracardiac repair, allowing simultaneous correction of defects in various locations or associated anomalies, with safety at least equal to or better than hypothermia. Extracorporeal circulation is now universally recognized as the preferred method for surgical treatment of atrial septal defects.
Operative Technique: Generally, a median sternotomy incision is used. For young women who prioritize cosmetic outcomes, bilateral submammary incisions can also be employed. The skin flaps above and below the incision are dissected, followed by a longitudinal or transverse sternotomy, and entry into the thorax through bilateral fourth intercostal incisions. Alternatively, entry can be achieved via a right anterior fifth intercostal incision. Upon opening the pericardium, the markedly enlarged right atrium, right ventricle, and pulmonary artery can be observed. A systolic thrill may also be palpable at the pulmonary artery. Attention should be paid to the presence of a left superior vena cava and any abnormalities in the entry of pulmonary veins into the left atrium. Pressing the right atrial wall with a finger often allows palpation of the atrial septal defect. Inserting a finger through a right atrial appendage incision for exploration can determine the location, size, and margin conditions of the atrial septal defect, as well as the normality of pulmonary vein and coronary sinus orifices and the presence of mitral or tricuspid regurgitation. Heparin is then administered, and the superior and inferior vena cavae are dissected and encircled with tapes. Cannulas for venous drainage are inserted through the right atrium and right atrial appendage, while an arterial cannula is inserted into the ascending aorta to establish cardiopulmonary bypass. Blood cooling is used to lower the body temperature to approximately 32°C. The ascending aorta is cross-clamped, and cold cardioplegic solution is injected into the aortic root, followed by cold saline into the pericardial cavity. The tapes around the superior and inferior vena cavae are tightened, and an oblique longitudinal incision is made anterior to the crista terminalis of the right atrium. Blood is aspirated from the right atrium to expose the atrial septum, but excessive aspiration should be avoided to prevent air from entering the left atrium and causing air embolism. The internal anatomy of the right atrium is carefully inspected, noting the location and size of the atrial septal defect, the integrity of the margin tissue, any abnormalities in the pulmonary vein orifices, and the conditions of the coronary sinus orifice, atrioventricular valves, and the orifices and valves of the superior and inferior vena cavae. For fossa ovalis defects with a diameter of less than 3 cm, direct continuous suturing can be performed (Figure 1), reinforced with a few interrupted sutures. The sutures should pass through sufficient atrial septal tissue at the anterior and posterior margins to ensure secure closure. For larger defects where direct continuous suturing would create excessive tension or where the atrial septal tissue at the margins is too fragile, a suitably sized and shaped Dacron patch or pericardial patch should be sutured to the defect margins. In adult cases, the tension from direct suturing may lead to postoperative atrial arrhythmias, making patch repair preferable (Figure 2). For cases with partial anomalous pulmonary venous drainage, sutures or patches are fixed to the atrial septal tissue at the right margin of the defect anterior to the pulmonary vein orifice, ensuring that after closure, pulmonary venous blood flows into the left atrium (Figure 3). Superior vena caval-type atrial septal defects are located near the superior vena cava orifice and often involve anomalous drainage of the right upper pulmonary vein into the right atrium. Care must be taken to avoid injury to the sinoatrial node when making the right atrial incision. This type of defect requires patch repair with pericardium or synthetic material rather than direct suturing to prevent stenosis or obstruction of the superior vena cava. The width of the patch should exceed the defect diameter by 50%, and its length should exceed the distance from the upper edge of the anomalous pulmonary vein orifice to the lower edge of the defect by 25%. This ensures that after repair, the interatrial communication is closed, the anomalous right pulmonary vein drains freely into the left atrium, and superior vena caval flow remains unobstructed (Figure 4). In cases where a small pulmonary vein anomalously drains high into the superior vena cava, only the atrial septal defect should be repaired, leaving the anomalous vein untreated to avoid obstruction of the superior vena cava by the patch. The residual small left-to-right shunt postoperatively does not significantly affect circulatory physiology. Some authors advocate right atrial plasty to enlarge the junction between the right atrium and the superior vena cava.
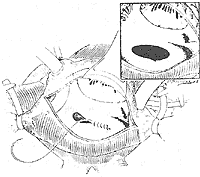
Figure 1 Suture of patent foramen ovale-type atrial septal defect
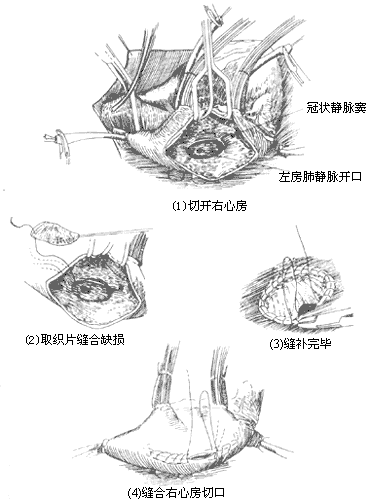
Figure 2 Patch repair of atrial septal defect
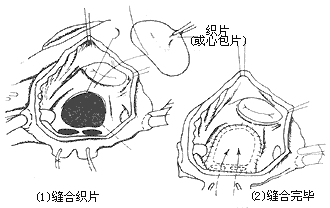
Figure 3 Repair of atrial septal defect with anomalous pulmonary venous return
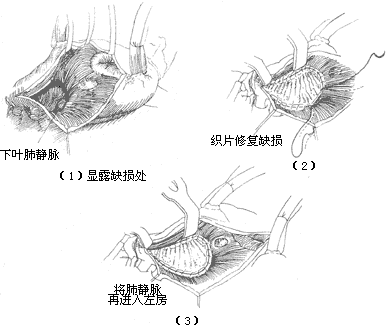
Figure 4 Repair of superior vena cava-type atrial septal defect
Inferior vena cava-type atrial septal defect: Generally, the defect area is large and located low. Most cases are suitable for patch repair. After exposing the right atrium during surgery, it is essential to identify the inferior vena cava valve to avoid mistakenly suturing it as the lower edge of the defect, which could lead to the inferior vena cava blood flowing back into the left atrium, causing a significant right-to-left shunt. Whether directly suturing or using a pericardial or synthetic patch for repair, the suture at the lower edge of the defect can pass through the atrial septal tissue on both sides of the defect and the posterior wall of the left atrium to prevent wrinkling of the posterior wall of the inferior vena cava. When suturing the lower edge of the defect, care must also be taken to avoid injuring the atrioventricular node and bundle (Figure 5). When the suture or patch repair of the atrial septal defect is nearing completion, a small catheter is inserted into the left atrium, and saline is injected into the left heart chamber. The tracheal tube is pressurized to inflate the lungs and expel any residual air in the left atrium before tying the final suture and closing the right atrial incision. The caval tourniquets are released, and thick needles are inserted into the left ventricular apex and the root of the main pulmonary artery to remove any residual air. The main pulmonary artery clamp is gradually released, and once the heart resumes normal beating and body temperature rises above 35°C, extracorporeal circulation is stopped. Postoperatively, blood samples from the superior vena cava, right atrium, or pulmonary artery are taken for oxygen content measurement to confirm the absence of residual left-to-right shunting. The caval and main pulmonary artery cannulas are removed as usual, and the pericardial incision is sutured. Drainage tubes are placed in the pericardial cavity and anterior mediastinum, and the sternum and chest wall incisions are closed. The surgical mortality rate for secundum-type atrial septal defects is generally 0–1%. After closing or suturing the defect, the left-to-right shunt disappears, the systolic murmur in the pulmonary valve area diminishes or disappears, cardiac function improves, and clinical symptoms resolve. In patients under 20 years old, postoperative chest X-rays show gradual reduction in heart size, but electrocardiograms may still show atrial arrhythmias, right bundle branch block, and right ventricular hypertrophy. Overall, the surgical outcomes are excellent, with long-term results similar to those of healthy individuals when treated in childhood. In adult patients with long-term right heart failure and pulmonary hypertension, surgical mortality increases. A few patients may develop sick sinus syndrome or conduction block due to intraoperative injury to the sinoatrial or atrioventricular nodes.
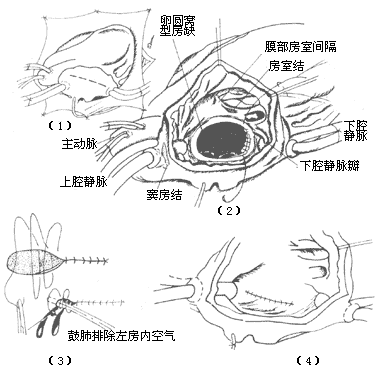
Figure 5 Patch repair of inferior vena cava-type atrial septal defect
(1) Incision (2) Exposure of the defect (3) Direct suturing; when nearing completion, a small catheter is inserted into the left atrium, and the tracheal tube is pressurized to inflate the lungs and expel residual air in the left atrium (4) Suturing the right atrial incision
Other cardiovascular diseases that produce left-to-right shunting at the atrial level, such as rupture of an aortic sinus aneurysm into the right atrium, coronary artery-right atrial fistula, anomalous pulmonary venous return, and rare cases of ventricular septal defect where blood shunts from the left ventricle to the right atrium, can generally be differentiated based on signs. If necessary, right heart catheterization, cardiac angiography, and echocardiography can be performed to confirm the diagnosis.





