| disease | Annular Pancreas |
| alias | Annluar Pancreas |
Annular pancreas is a congenital developmental anomaly where a band of pancreatic tissue partially or completely encircles the first or second segment of the duodenum, leading to luminal narrowing. This condition was first identified during an autopsy by Tiedemann in 1818 and was first reported by Ecker in 1862. To date, there have been only over 300 cases reported in the global literature. In 1981, Ding Shihai summarized 56 cases from the literature in China, with a male to female ratio of 2:1.
bubble_chart Etiology
The exact cause of the formation of annular pancreas during embryonic development is not yet fully understood, and there are many theories, with two main explanations. The pancreas develops from several protrusions on the wall of the primitive gut of the embryo. The dorsal pancreatic bud arises directly from the wall of the duodenum, while the ventral pancreatic bud originates from the base of the hepatic diverticulum. Later, the dorsal pancreatic bud develops into the body and tail of the pancreas, with its stalk becoming the accessory pancreatic duct; the stalk of the ventral pancreatic bud becomes the main pancreatic duct, and its end forms the head of the pancreas. Around the 6th week of embryonic development, as the duodenum rotates, the ventral pancreas also moves to the posterior side of the dorsal pancreas; by the 7th week, the dorsal and ventral pancreases begin to make contact, eventually merging into a single pancreas, with the two pancreatic ducts also fusing and connecting. Therefore, one theory suggests that annular pancreas results from the failure of the ventral pancreatic bud to rotate with the duodenum and fuse with the dorsal pancreatic bud; another theory proposes that simultaneous hypertrophy of the ventral and dorsal pancreatic buds leads to the formation of annular pancreas, which completely or partially encircles the second part of the duodenum, causing obstruction.
bubble_chart Pathological ChangesThe annular pancreas is generally about 1 cm wide and encircles the second part of the duodenum. Based on the extent of encirclement, it can be classified into complete annular pancreas and incomplete annular pancreas, with the latter being more common, where the annular pancreas only partially encircles the duodenum, accounting for about 2/3 to 4/5 of the intestinal circumference.
The annular pancreas is often true pancreatic tissue, containing normal acini and islet tissue, but in some patients, it may consist only of fibrous tissue. The ducts within the annular pancreas may not communicate with the main pancreatic duct and may open separately into the duodenum.
bubble_chart Clinical Manifestations
Clinically, annular pancreas is often classified into neonatal and adult types, with clinical manifestations closely related to the degree of duodenal compression and accompanying pathological changes.
(1) The neonatal type usually manifests within the first week after birth, with rare cases occurring after two weeks. It primarily presents as acute complete duodenal obstruction. Affected infants exhibit persistent vomiting, with bile present in the vomitus. Frequent vomiting can lead to dehydration, electrolyte imbalance, acid-base disturbance, and malnutrition. In cases of incomplete duodenal obstruction, symptoms include intermittent abdominal pain and vomiting, often accompanied by upper abdominal fullness and discomfort, which worsens after eating. These symptoms may recur. Additionally, annular pancreas is often associated with other congenital anomalies such as Down syndrome, esophageal atresia, tracheoesophageal fistula, Meckel's diverticulum, congenital heart disease, and clubfoot.
(2) The adult type is more common in individuals aged 20 to 40 and typically presents with symptoms of chronic incomplete duodenal obstruction. The earlier the symptoms appear, the more severe the duodenal obstruction. Patients mainly experience recurrent upper abdominal pain and vomiting, which occur in paroxysmal episodes. Abdominal pain worsens after eating and may be relieved after vomiting, with the vomitus containing gastric and duodenal fluids, including bile. Besides duodenal obstruction, patients may also develop other pathological changes, leading to corresponding clinical symptoms.1. Peptic ulcer
The incidence of gastric and duodenal ulcers in patients with annular pancreas can reach 30-40%, with duodenal ulcers being more common. The development of ulcers may be related to the compression by the annular pancreas, prolonged gastric fluid retention, and high acidity of gastric and duodenal contents.
2. Acute pancreatitis
The occurrence of pancreatitis in patients with annular pancreas ranges from 15-30%, possibly due to abnormalities in the pancreatic duct system, pancreatic fluid stasis, or bile reflux into the pancreatic duct. Pancreatitis may be limited to the annular pancreas or involve the entire pancreas. Edema from acute pancreatitis or fibrous scarring from chronic pancreatitis can exacerbate duodenal obstruction.
3. Biliary obstruction
Clinically less common, biliary obstruction can occur due to the annular pancreas located at the ampulla of Vater, significant narrowing of the second part of the duodenum caused by the annular pancreas compressing the common bile duct, or pancreatitis, leading to jaundice. Prolonged cases may also result in secondary biliary stones.
bubble_chart Auxiliary Examination1. X-ray examination: The abdominal plain film mainly shows duodenal obstruction. The supine film reveals distension and gas in the stomach and duodenal bulb, known as the double bubble sign; if there is a large amount of retained fluid in the stomach and duodenal bulb, a fluid level can be seen in both the stomach and duodenal bulb on the upright film (Figure 1).
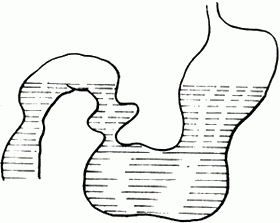
Figure 1 Double fluid levels in the stomach and duodenum
2. Barium meal examination: It shows gastric distension and ptosis, with a large amount of fasting retained fluid in the stomach and prolonged emptying time; the duodenal bulb is symmetrically enlarged and elongated, with a smooth and rounded lower edge; in the descending part of the duodenum, occasionally in the first or third part, there is a localized narrow area with neat edges, and the mucosal folds in this area are sparse, with reverse peristalsis visible in the intestinal tract above the narrow area (Figure 2)
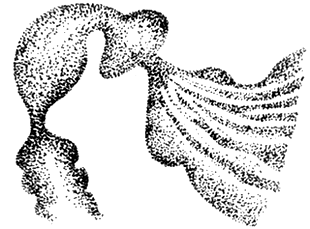
Figure 2 Narrowing of the descending part of the duodenum
The diagnosis of annular pancreas is not straightforward. Based on typical symptoms and signs, combined with X-ray findings, the possibility of this condition should be considered. However, some cases are only definitively diagnosed during surgery.
bubble_chart Treatment Measures
For annular pancreas without symptoms or with inconspicuous symptoms, surgery may not be necessary. However, if it has caused duodenal stenosis or obstruction, surgical treatment is mandatory. The surgical methods are mainly divided into two categories:
1. Resection or excision of the annular pancreas
If the annular pancreatic tissue is thin, with sparse vascular distribution and not tightly adhered to the intestinal wall, the annular pancreas can be severed, or partially or completely removed to relieve duodenal obstruction. If the duodenum has developed stenosis due to long-term compression, a longitudinal incision and transverse suture of the duodenum can be performed to enlarge the intestinal lumen. The disadvantage of this procedure is that it may cause complications such as pancreatic injury, pancreatic fistula, pancreatic cyst, or duodenal fistula; sometimes, the stenosis or obstruction of the duodenum may not be completely relieved after surgery. Therefore, this method is generally not recommended at present.
2. Bypass surgery
(1) Duodeno-duodenal side-to-side anastomosis: This procedure is relatively easy to perform, can completely relieve duodenal obstruction, maintain gastric function, and does not risk injuring the pancreatic duct or causing pancreatic fistula, making it more physiological and the preferred method. Surgical method: After incising the posterior membrane of the lateral edge of the duodenum, free the proximal and distal ends of the obstructed duodenum; then place two traction sutures on the anterior wall of the intestinal tube at the proximal and distal ends of the obstruction, make a transverse incision on the anterior wall of the proximal intestinal tube, a longitudinal incision on the anterior wall of the distal end, and perform interrupted full-thickness sutures with No. 1 silk thread, followed by interrupted seromuscular layer sutures.
(2) Duodenojejunal Roux-en-Y anastomosis (anterior or posterior to the colon): This procedure has the advantages of duodeno-duodenal side-to-side anastomosis, but the following points should be noted during the operation: ① The anastomosis should be chosen at the lowest point of the proximal end of the duodenal obstruction to avoid forming a blind loop. ② The anastomosis should not be too small to avoid stenosis. ③ The jejunum should not be twisted or angled during anastomosis to avoid obstruction. ④ The jejunum is severed 15-20 cm from the ligament of Treitz, and the distal end is anastomosed to the lowest point of the proximal end of the duodenal obstruction.
(3) Duodenojejunal side-to-side anastomosis: The advantages of this procedure are the same as those of duodeno-duodenal side-to-side anastomosis. The method involves anastomosing a segment of jejunum 15-20 cm from the ligament of Treitz, anterior or posterior to the transverse colon, side-to-side to the proximal end of the obstructed duodenum. The precautions during surgery are basically the same as those for duodenojejunal Roux-en-Y anastomosis.
(4) Gastrojejunostomy: This procedure has two prominent features: ① Postoperative anastomotic edge ulcers may occur. ② The proximal duodenal drainage is poor, and duodenal obstruction cannot be well relieved. Therefore, except when there are tight adhesions around the duodenum that prevent other bypass surgeries, this procedure is generally not recommended. Sanford believes that performing a subtotal gastrectomy followed by gastrojejunostomy is the most ideal method for adult cases of annular pancreas.
In addition, for patients with biliary obstruction, besides relieving duodenal obstruction, biliary obstruction must also be relieved. A subtotal gastrectomy, Billroth-II anastomosis, plus choledocho-duodenal end-to-side anastomosis can be performed. For annular pancreas combined with gastric and duodenal obstruction, a subtotal gastrectomy, Billroth-II anastomosis, and, if necessary, vagotomy can be performed.
Tongue-tie, esophageal atresia, tracheoesophageal fistula, Meckel's diverticulum, congenital heart disease, clubfoot, peptic ulcer, acute pancreatitis, biliary obstruction, etc.
When considering annular pancreas, it should be differentiated from the following diseases.
1. Congenital duodenal atresia
Occasionally seen in newborns, the lesion is located in the descending part of the duodenum, with frequent vomiting after birth, and the vomitus may contain bile. Barium contrast imaging shows complete obstruction of the duodenum with no gas in the lower intestinal tract. During surgery, no pancreatic tissue is found surrounding the descending part of the duodenum.
2. Congenital hypertrophic pyloric stenosis
Mostly presents with regurgitation and vomiting several weeks after birth, with no bile in the vomitus. The upper abdomen is distended, and gastric peristaltic waves may be visible. In 95-100% of cases, an olive-shaped mass can be palpated in the right upper abdomen. Barium contrast imaging shows gastric dilation, narrowing and elongation of the pyloric canal, and delayed gastric emptying.
3. Superior mesenteric artery syndrome
This condition refers to chronic obstruction of the third or fourth part of the duodenum caused by compression from the superior mesenteric artery. Main symptoms include upper abdominal fullness and discomfort, intermittent vomiting with bile in the vomitus. Barium contrast imaging shows significant obstruction and dilation of the duodenum, with blockage at the third or fourth part of the duodenum.
4. Tumors of the pancreatic head or ampulla of Vater
Patients with annular pancreas accompanied by jaundice, especially elderly patients, should be differentiated from tumors of the pancreatic head or duodenal papilla. The latter shows duodenal loop enlargement, compression and deformation of the inner edge of the descending part, destruction of mucosal folds, filling defects, inverted "3" sign, and double contour sign on barium contrast imaging.
In addition, it should also be differentiated from congenital biliary atresia, duodenal diverticulum, and low duodenal ulcer.





