| disease | Injury-induced Shock |
| alias | Traumatic Shock |
Severe trauma, especially when accompanied by a certain amount of bleeding, often leads to shock, known as traumatic shock. Traumatic shock is commonly seen in patients who have suffered severe injuries, such as fractures, crush injuries, and major surgeries. The loss of plasma or whole blood from the body, combined with bleeding at the injury site, edema, and fluid exudation into the tissue spaces that cannot participate in circulation, can significantly reduce the circulating blood volume. Additionally, the gradual necrosis or decomposition of injured tissues produces protein breakdown products with vasodepressive effects, such as histamine and proteases, which cause microvascular dilation and increased vascular permeability, further reducing the effective circulating blood volume and exacerbating tissue ischemia.
bubble_chart Pathogenesis
Trauma can trigger a series of pathophysiological changes. The fundamental change is the uneven distribution of body fluids. Peripheral blood vessels may dilate, cardiac output function may be normal, or even compensatory increase, but tissue perfusion pressure is insufficient.
Traumatic shock can be accompanied by hypovolemic shock due to loss of blood and fluid loss. This includes the effects of chemical mediators, injury factors, oxygen free radicals, toxic substances, and neuroendocrine changes, which enhance the permeability of microvessels, leading to exudation. This is hypovolemic shock caused by vascular injury exudation, so it should be classified as vasogenic shock. Although it is hypovolemic shock, it is different from hypovolemic shock caused by loss of blood. Traumatic shock is accompanied by a large amount of fluid loss, and a large amount of fluid is isolated in the extravascular space, which further activates inflammatory mediators and can develop into acute inflammatory response syndrome (SIRS).
Microcirculation disorders (ischemia, congestion, disseminated intravascular coagulation) lead to insufficient microcirculation stirred pulse blood perfusion, and important life organs suffer from functional and metabolic disorders due to hypoxia, which is a common pattern of all types of shock. The changes in microcirculation during shock can be roughly divided into the late stage [third stage], namely the microcirculation ischemia phase, the microcirculation congestion phase, and the microcirculation coagulation phase (for details, see loss of blood shock).The progression from the microcirculation congestion phase to the microcirculation coagulation phase is a manifestation of shock deterioration. Its characteristics are: on the basis of microcirculation congestion, fibrin thrombi form in the microcirculation (especially at the venous end of capillaries, venules, and small veins), and there are often focal or diffuse hemorrhages; tissue cells undergo degeneration and necrosis due to severe hypoxia.
Disseminated intravascular coagulation is closely related to shock, so how does shock cause disseminated intravascular coagulation?
1. The stress response increases blood coagulability. The causes of shock (such as trauma, burns, hemorrhage, etc.) and shock itself are strong stimuli that can cause a stress response, sympathetic nerve excitation, and increased pituitary-adrenal cortex activity, leading to an increase in platelets and coagulation factors in the blood, enhanced platelet adhesion and aggregation ability, providing the necessary material basis for coagulation.
2. Release and activation of coagulation factors. Some causes of shock (such as trauma, burns, etc.) themselves can cause the release and activation of coagulation factors. For example, injured tissues can release a large amount of tissue thromboplastin, initiating the extrinsic coagulation process; large-area burns cause massive destruction of red blood cells, and the phospholipids in the red blood cell membrane and ADP released from the destruction of red blood cells promote the coagulation process.
3. Microcirculation disorders, tissue hypoxia, and local increases in histamine, kinins, lactic acid, etc. These substances on one hand cause capillary dilation and congestion, increased permeability, slow blood flow, blood concentration, increased red blood cell viscosity, which is conducive to thrombus formation; on the other hand, they damage capillary endothelial cells, expose collagen, activate coagulation factor XII, and cause platelet adhesion and aggregation.4. Hypoxia reduces the function of the mononuclear phagocyte system, which cannot promptly clear prothrombinase, thrombin, and fibrin. As a result, under the influence of the above factors, disseminated intravascular coagulation occurs (Figure 1).
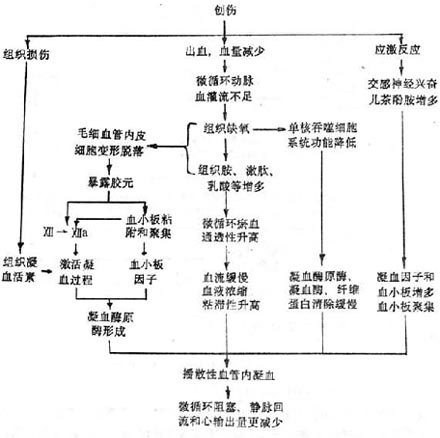
Figure 1 Mechanism of traumatic shock causing disseminated intravascular coagulation
It should be noted that in different types of shock, the timing of disseminated intravascular coagulation formation may vary. For example, in burn and traumatic shock, due to massive tissue destruction, and in septic shock, due to the direct injury of internal toxins to vascular endothelium, disseminated intravascular coagulation can occur earlier, while in loss of blood shock, disseminated intravascular coagulation occurs later.
bubble_chart Clinical Manifestations
Clinical Manifestations According to the progression of shock, it can be divided into two stages: the compensatory stage and the inhibitory stage of shock, also known as the pre-shock stage or the shock stage.
1. Compensatory Stage of Shock In cases of trauma accompanied by bleeding, when the loss of blood volume does not exceed 20%, due to the body's compensatory mechanisms, the patient's central nervous system excitability increases, and sympathetic nerve activity intensifies. Manifestations include mental tension or dysphoria, pale complexion, cold and clammy extremities, accelerated heart rate, hyperventilation, etc. Blood pressure is normal or slightly elevated, with an increase in diastolic pressure reflecting the contraction of small stirred pulses, leading to a narrowed pulse pressure. Urine output is normal or reduced. At this stage, if managed appropriately, shock can be quickly corrected. If not managed properly, the condition progresses to the inhibitory stage.
2. Inhibitory Stage of Shock The patient exhibits apathy, sluggish responses, and may even experience unconsciousness, cyanosis of the lips, cold sweating, thready and rapid pulse, decreased blood pressure, and further narrowing of the pulse pressure difference. In severe cases, the skin and mucous membranes show significant cyanosis, the extremities are cold, the pulse is difficult to palpate, blood pressure is unmeasurable, and there is no urine output. Metabolic acidosis may also occur. The appearance of ecchymosis on the skin and mucous membranes or gastrointestinal bleeding indicates that the condition has progressed to the stage of disseminated intravascular coagulation. Progressive dyspnea, rapid pulse, dysphoria, cyanosis, or coughing up pink frothy sputum, with the oxygen partial pressure of stirred pulse blood dropping below 8kPa (60mmHg), and symptoms and oxygen partial pressure not improving despite high oxygen administration, often suggest the presence of respiratory distress syndrome.The clinical manifestations of shock generally change with the progression of the condition.
bubble_chart Auxiliary Examination
1. Central Venous Pressure The venous system accommodates 55-60% of the total blood volume. Changes in central venous pressure generally occur earlier than changes in stirred pulse pressure. It is influenced by many factors, mainly: ① blood volume; ② venous vascular tension; ③ right ventricular ejection capacity; ④ intrathoracic or pericardial pressure; ⑤ venous return heart blood volume. The normal value of central venous pressure is 0.49~0.98kPa (5~10cmH2O). In cases of hypotension, a central venous pressure below 0.49kPa (5cmH2O) indicates insufficient blood volume; above 1.47kPa (15cmH2O), it suggests cardiac insufficiency, excessive venous vascular bed contraction, or increased pulmonary circulation resistance; above 1.96kPa (20cmH2O), it indicates congestive heart failure. Continuous measurement of central venous pressure and observation of its changes are more reliable than a single measurement.
2. Pulmonary Stirred Pulse Wedge Pressure Central venous pressure cannot directly measure the pressure of pulmonary veins, left atrium, and left ventricle. Therefore, before the central venous pressure rises, the left heart pressure may have already increased, but it cannot be detected by central venous pressure measurement. Using a Swan-Ganz pulmonary stirred pulse floating catheter, after inserting it from a peripheral vein into the superior vena cava, inflate the balloon to allow it to flow through the right atrium, right ventricle, and into the pulmonary stirred pulse, measuring the pulmonary stirred pulse pressure and pulmonary stirred pulse wedge pressure, can provide information on the pressure of pulmonary veins, left atrium, and left ventricle at the end of diastole, reflecting the status of pulmonary circulation resistance. The normal value of pulmonary stirred pulse pressure is 1.3~2.9kPa. The normal value of pulmonary stirred pulse wedge pressure is 0.8~2.0kPa, an increase indicates increased pulmonary circulation resistance. In pulmonary edema, the pulmonary stirred pulse wedge pressure exceeds 4.0kPa. When the pulmonary stirred pulse wedge pressure has increased, even if the central venous pressure has not increased, excessive fluid infusion should be avoided to prevent pulmonary edema, and measures to reduce pulmonary circulation resistance should be considered. Through pulmonary stirred pulse catheterization, blood can be drawn for mixed venous blood qi aspect analysis to understand the pulmonary arteriovenous shunt situation, that is, the degree of change in the ventilation/perfusion ratio of the lungs. The application of the catheter has certain complications. Therefore, it is only used when necessary in the rescue of severe shock patients. The catheter should not be left in the pulmonary stirred pulse for more than 72 hours.
3. Cardiac Output and Cardiac Index In shock, cardiac output generally decreases. However, in septic shock, cardiac output can be higher than normal, so it is necessary to measure it to guide treatment. Through pulmonary stirred pulse catheterization and thermodilution method, cardiac output can be measured and cardiac index calculated. The normal value of cardiac index is 3.20±0.20/L(min﹒m2).
4. Stirred Pulse Blood Qi Aspect Analysis Stirred pulse blood oxygen partial pressure (PaO2) normal value is 10~13.3kPa (75~100mmHg), stirred pulse carbon dioxide partial pressure (PaCO2) normal value is 5.33kPa (40mmHg), stirred pulse blood pH normal value is 7.35~7.45. In shock, if the patient has no pre-existing lung disease, due to frequent hyperventilation, PaCO2 is generally lower or within the normal range. If it exceeds 5.9~6.6kPa (45~50mmHg) with good ventilation, it is often a sign of severe pulmonary insufficiency. PaO2 below 8.0kPa (60mmHg), with no significant increase after inhaling pure oxygen, is often a signal of respiratory distress syndrome. Through blood qi aspect analysis, the evolution of metabolic acidosis during shock can also be understood.
5. Stirred pulse blood lactate measurement The normal value is 1-2 mmol/L. Generally speaking, the longer the duration of shock and the more severe the blood perfusion disorder, the higher the stirred pulse blood lactate concentration. A continuous increase in lactate concentration indicates a serious condition and a poor prognosis. For those with a lactate concentration exceeding 8 mmol/L, the mortality rate is almost 100%.
6. Laboratory Tests for Disseminated Intravascular Coagulation For patients suspected of having disseminated intravascular coagulation, tests should be conducted to assess the extent of platelet and coagulation factor consumption, as well as tests reflecting fibrinolytic activity. A platelet count below 80×109/L, fibrinogen less than 1.5g/L, prothrombin time prolonged by more than 3 seconds compared to normal, and a positive paracoagulation test can confirm the diagnosis of disseminated intravascular coagulation.
The diagnosis of hypovolemic shock is generally not difficult. What is important is to make an early diagnosis. Waiting until blood pressure drops to diagnose shock may sometimes be too late. Whenever there is a significant loss of blood, dehydration, or severe trauma, the possibility of shock should be considered. During observation, if the patient shows signs of mental excitement, dysphoria, restlessness, cold sweating, increased heart rate, narrowed pulse pressure, and decreased urine output, shock should be considered. If the patient experiences unquenchable thirst, apathy, sluggish response, pale skin, cold sweating, cold extremities, shallow and rapid breathing, thready pulse, systolic blood pressure dropping below 12 kPa (90 mmHg), and oliguria, it should be considered that the patient has entered the inhibitory phase of shock. As for the diagnosis of septic shock, it can be considered based on the presence of severe infection in the patient, along with some clinical manifestations of the compensatory phase of shock, or the sudden appearance of obvious hyperventilation. High-resistance septic shock has some common manifestations of shock, making diagnosis not difficult. However, low-resistance septic shock patients lack these common manifestations, making diagnosis more difficult and requiring some special tests to confirm the diagnosis.
Monitoring of Shock Through monitoring shock patients, the diagnosis can be further confirmed, and the condition can be better judged and treatment guided.
(1) General Monitoring Often can determine the presence and progression of shock.
1. Mental State Can reflect the perfusion of brain tissue. A clear mind and good response in the patient indicate sufficient circulating blood volume. Apathy or dysphoria, dizziness, blurred vision, or syncope when changing from lying to sitting position often indicate insufficient circulating blood volume and the continued presence of shock.
2. Limb Temperature and Color Reflect the perfusion of the body surface. Warm limbs, dry skin, temporary local ischemia turning pale when lightly pressing the nail or lip, and quickly turning red upon release indicate improvement in shock. In shock, the skin of the limbs is often pale and cold; lightly pressing the nail or lip turns it pale, and it slowly returns to red upon release.
3. Blood Pressure During the compensatory phase of shock, intense vasoconstriction can maintain or keep blood pressure close to normal. Therefore, blood pressure should be measured regularly and compared. A gradual drop in blood pressure, with systolic pressure below 12 kPa (90 mmHg) and pulse pressure less than 2.67 kPa (20 mmHg), is evidence of shock. A rise in blood pressure and an increase in pulse pressure indicate improvement in shock.
4. Pulse Rate A thready and rapid pulse often appears before blood pressure drops. Sometimes, even if blood pressure remains low, a clear pulse and warm hands and feet often indicate that shock is improving. The shock index (pulse rate/systolic blood pressure [in mmHg]) can help determine the presence and severity of shock. An index of 0.5 generally indicates no shock; over 1.0 to 1.5 indicates the presence of shock; above 2.0 indicates severe shock.
5. Urine Output Is an indicator of renal blood perfusion and can also reflect the perfusion of vital organs. A urinary catheter should be placed to observe hourly urine output. Urine output less than 25 ml per hour with increased specific gravity indicates continued renal vasoconstriction or insufficient blood volume; normal blood pressure but still low urine output with decreased specific gravity may indicate acute renal failure. Stable urine output above 30 ml per hour indicates correction of shock.
(2) Special Monitoring The pathophysiological changes of shock are very complex. In severe or prolonged hypovolemic shock and septic shock, hemodynamic changes often cannot be fully reflected by the above monitoring items, and further special monitoring items are needed to better judge the condition and take correct treatment measures (see laboratory tests).
bubble_chart Treatment Measures
Although trauma-induced shock is hypovolemic in nature, fluid resuscitation often yields suboptimal results, such as in cases of severe large-area burns and extensive soft tissue injuries. This is typically due to increased microvascular permeability caused by microcirculatory disturbances, leading to excessive fluid requirements. Despite aggressive resuscitation efforts, multiple organ dysfunction may still occur. Therefore, traumatic shock often requires invasive monitoring with a pulmonary artery catheter; mechanical ventilation during treatment; and pharmacological support for the cardiovascular system, such as inotropic agents like dobutamine or dopamine. Additionally, enhanced protection of vital organs is necessary.
Traumatic shock is fundamentally hypovolemic shock, but changes and impacts on the cardiovascular system should not be overlooked. In other words, post-trauma, cardiac function may be suppressed, and vasoconstriction may alter. Cardiovascular support medications can be used. Notably, vasoconstrictors should not be administered to patients with poor peripheral circulation, such as those with pale complexion, cold and clammy skin, or cyanotic patches.
Additionally, trauma can induce neuropsychiatric factors. It is also important to note that trauma is often accompanied by internal or external bleeding, and the post-injury course may involve infection. Thus, traumatic shock may be linked to hemorrhagic shock and septic shock. In clinical diagnosis, condition assessment, and treatment, comprehensive analysis and careful consideration are essential for proper management. Such situations are more common in car accidents, falls, and combat injuries. Due to the complexity of multiple or combined injuries and the variable nature of the condition, treatment is challenging and requires full attention. Traumatic shock can lead to systemic and local immune suppression, increasing susceptibility to infection. Antibiotics and immune-enhancing medications should be administered.
For local trauma conditions, surgical intervention should be performed at an appropriate time as needed.
1. Actively prevent and treat infections;
2. Properly handle on-site trauma treatment, such as timely hemostasis, analgesia, and warmth preservation.
3. For patients with loss of blood or excessive fluid loss (such as vomiting, diarrhea, hemoptysis, gastrointestinal bleeding, excessive sweating, etc.), timely fluid replacement or blood transfusion should be considered as appropriate.




