| disease | Persistent Truncus Arteriosus |
Persistent truncus arteriosus refers to a condition where both the left and right ventricles eject blood into a common truncus arteriosus. The semilunar valve of the truncus arteriosus straddles a high ventricular septal defect. Anatomically, only the common trunk is visible, with no remnants of an atretic aorta or pulmonary artery. The systemic, pulmonary, and coronary circulations all directly receive blood from the truncus arteriosus. Persistent truncus arteriosus is an extremely rare and complex congenital cardiovascular malformation, with an incidence of approximately 0.5%, accounting for about 1-3% of congenital cardiovascular anomalies in autopsies.
bubble_chart Pathological Changes
During the 3rd and 4th weeks of embryonic development, under normal circumstances, the development of the stirred pulse trunk septum divides the stirred pulse trunk into the ascending aorta and the main pulmonary artery. The stirred pulse trunk septum grows spirally from the conus towards the cephalic direction, positioning the ascending aorta posteriorly and to the left, and the main pulmonary artery anteriorly and to the right. The stirred pulse trunk septum connects with the conal septum of the conus, participating in the formation of the membranous part of the ventricular septum, thereby closing the interventricular foramen. If the bulbar septum is absent or underdeveloped, a high ventricular septal defect forms, and the stirred pulse trunk overrides the ventricular septal defect. The semilunar valves of the stirred pulse trunk usually consist of three cusps, but there can be malformations with 2 to 6 cusps. The stirred pulse duct is often absent, and even if present, it is functionally insignificant. The pulmonary artery may branch from the root, trunk, or arch of the stirred pulse trunk, or the pulmonary artery may not develop at all, with pulmonary circulation blood coming only from enlarged bronchial arteries. Therefore, persistent stirred pulse trunk can manifest in various types, but regardless of the type, the blood for systemic, pulmonary, and coronary circulation comes from the ventricles and the stirred pulse trunk.
Hemodynamics: In cases of persistent stirred pulse trunk, all blood from the left and right ventricles enters the stirred pulse trunk. Venous blood and oxygenated blood from the pulmonary circulation ejected by the left ventricle mix with blood from the systemic circulation ejected by the right ventricle in the stirred pulse trunk. The degree of reduced oxygen saturation depends on the amount of pulmonary blood flow. If pulmonary blood flow is high, clinical cyanosis is not obvious or mild, but increased cardiac load combined with stirred pulse valve membrane incompetence can easily lead to heart failure. Elevated left atrial pressure can cause pulmonary edema. If pulmonary blood flow is low, cyanosis is pronounced, leading to reduced pulmonary blood flow. The most common cause is the gradual development of pulmonary vascular obstructive disease due to the pulmonary vascular bed bearing high systemic pressure, leading to increased pulmonary vascular resistance and reduced blood flow. Pulmonary artery stenosis can also cause reduced pulmonary blood flow but is less common.bubble_chart Clinical Manifestations
According to the different origins of the pulmonary stirred pulse, there are several classification methods for persistent stirred pulse trunk. Currently, the clinical classification commonly used is the four types according to the Collect and Edwards method (Figure 1).
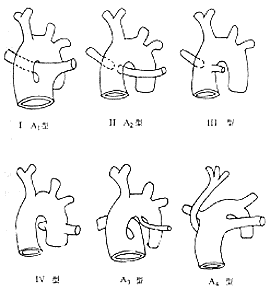
Figure 1 Schematic diagram of the anatomical classification of persistent stirred pulse trunk
Type I: The stirred pulse trunk is partially separated, and the main pulmonary stirred pulse originates from the proximal end of the stirred pulse trunk, located on the left side and in the same plane as the right ascending aorta, receiving blood from both ventricles. This type is common, accounting for about 48%.
Type II: The left and right pulmonary stirred pulses share a common opening or are close to each other, originating from the posterior wall of the middle part of the stirred pulse trunk, accounting for about 29%.Type III: The left and right pulmonary stirred pulses originate separately from both sides of the stirred pulse trunk, accounting for about 11%.
Type IV: The pulmonary stirred pulse originates from the descending thoracic aorta or the pulmonary stirred pulse is absent, with the pulmonary blood supply coming from the bronchial stirred pulse, accounting for about 12%.
Van Praagh classified the common stirred pulse trunk into four categories based on the degree of formation of the aortic-pulmonary stirred pulse septum and the anatomical morphology of the pulmonary stirred pulse and aortic arch:
Type A1: Accounts for about 50%, with partial formation of the stirred pulse trunk septum but a large defect above the valve. A short main pulmonary stirred pulse originates from the left dorsal side of the stirred pulse trunk and divides into left and right pulmonary stirred pulses. The stirred pulse trunk itself becomes the ascending aorta, with about 7% of cases having stenosis at the origin of the main pulmonary stirred pulse.
Type A1-A2 Transition: Accounts for about 9%, indistinguishable in both heart blood vessel angiography and surgery.
Type A2: Accounts for about 21%, with absence of the aortic-pulmonary stirred pulse septum and the main pulmonary stirred pulse trunk. Both pulmonary stirred pulses originate directly from the dorsal or lateral side of the stirred pulse trunk and travel towards the lungs. Their openings may be separate or close together, with reduced pulmonary perfusion due to stenosis or hypoplasia at the origin of the pulmonary stirred pulse branches.
Type A3: Accounts for about 8%, with only a single pulmonary stirred pulse branch originating above the sinus of Valsalva of the stirred pulse trunk, supplying the ipsilateral lung lobe, while the other lung lobe is supplied by a major aortopulmonary collateral or a pulmonary stirred pulse originating from the aortic arch or descending aorta. The absent pulmonary stirred pulse is usually on the same side as the aortic arch, rarely on the opposite side.Type A4: Accounts for about 12%, with the stirred pulse trunk directly dividing above the valve into a narrow or hypoplastic ascending aorta and a significantly enlarged main pulmonary stirred pulse trunk. A large stirred pulse duct connects the pulmonary stirred pulse branch to the descending aorta, with the hypoplastic aortic arch having stenosis at the isthmus or even complete discontinuity.
Clinical manifestations: In the first few weeks after birth, due to high pulmonary vascular resistance and low pulmonary blood flow, clinical symptoms are not obvious. As pulmonary vascular resistance decreases, symptoms of heart failure and pulmonary infection may appear. Increased pulmonary blood flow often presents with dyspnea, heart failure, and tachycardia. Decreased pulmonary blood flow results in cyanosis, accompanied by polycythemia and clubbing of fingers (toes). Physical examination: The patient's general condition is weak, with no weight gain, increased heart rate, enlarged heart, hepatomegaly, and a single second heart sound heard in the pulmonary valve area. A loud, rough systolic murmur and thrill are heard at the third and fourth intercostal spaces along the left sternal border. In cases with valve insufficiency, an early or intermediate stage diastolic murmur is heard at the apex. Pulmonary valve insufficiency often presents with a water-hammer pulse. Chest X-ray shows an enlarged heart shadow, increased pulmonary vascular markings, mainly ventricular enlargement, and a significantly widened and strongly pulsating ascending aorta without visible pulmonary artery. About 25% of cases have a right-sided aorta. The origin of the pulmonary artery is higher than normal, and if the pulmonary artery branch shadow reaches the level of the aortic arch, it has diagnostic value. Electrocardiogram: In cases of increased pulmonary blood flow, there is hypertrophy of the left and right ventricles and increased pulmonary vascular resistance. In cases of decreased pulmonary blood flow, there is right ventricular hypertrophy. Echocardiography: The pulmonary artery trunk is seen straddling the ventricular septal defect, with common left atrial and left ventricular enlargement, and possible thickening of the pulmonary valve. Cardiac catheterization: Increased right ventricular pressure, similar systolic pressures in the left and right ventricles, and similar pressures in the pulmonary artery and pulmonary trunk. The catheter can enter the brachiocephalic branch of the aortic arch from the right ventricle. Angiography shows a single pulmonary artery trunk straddling the ventricular septal defect with only one set of semilunar valves. Both the coronary artery and pulmonary artery originate from the pulmonary trunk.
bubble_chart Treatment Measures
Through a median sternotomy incision, the procedure is performed under cardiopulmonary bypass. However, before the main pulmonary artery is clamped during the extracorporeal circulation, the left and right pulmonary arteries must be clamped first to prevent acute pulmonary edema. Then, the pulmonary artery is severed from the main pulmonary artery. If the pulmonary artery has two separate openings, both openings are excised along with a portion of the main pulmonary artery wall. The defect in the posterior wall of the pulmonary artery and the ventricular septal defect are repaired with a patch, ensuring that the pulmonary artery communicates only with the left ventricle. A valved external conduit is used to establish a connection between the right ventricle and the pulmonary artery.
Most infants die during infancy. The primary cause of death is heart failure, with other causes including brain abscess and endocarditis. The surgical mortality rate is high, and the prognosis is poor.
(1) Pulmonary stirred pulse In severe tetralogy of Fallot of the closed type, cyanosis and heart failure appear immediately after birth. X-ray examination shows pulmonary ischemia and enlargement of the right atrium and ventricle. The electrocardiogram indicates hypertrophy of the right atrium and ventricle. Right heart catheterization reveals increased pressure in the right atrium and ventricle. Doppler echocardiography and heart blood vessel angiography can confirm the diagnosis.
(2) Main and pulmonary stirred pulse septal defect Similar to type I of persistent stirred pulse trunk, echocardiography can reveal two sets of large stirred pulse valve membranes, and main stirred pulse angiography can display the two large stirred pulses and the location of the septal defect.
(3) Large stirred pulse transposition Cyanosis and heart failure appear immediately after birth. If accompanied by a large atrial septal defect or ventricular septal defect, the cyanosis is milder and symptoms appear later. X-ray examination shows an egg-shaped enlargement of the heart, and heart blood vessel angiography reveals that the main stirred pulse originates from the right ventricle, while the main pulmonary stirred pulse originates from the left ventricle.
(4) Tricuspid atresia The right atrium is enlarged, and the left ventricle is hypertrophied. The electrocardiogram shows a left axis deviation, whereas persistent stirred pulse trunk commonly shows right ventricular hypertrophy. Doppler echocardiography and heart blood vessel angiography can reveal tricuspid atresia.





