| disease | Intestinal Cancer |
Rectal cancer refers to cancer occurring between the dentate line and the rectosigmoid junction, being one of the most common malignant tumors in the digestive tract. Due to its low position, rectal cancer is easily diagnosed by digital rectal examination and sigmoidoscopy. However, because of its deep location within the pelvis and complex anatomical relationships, complete surgical removal is challenging, and the postoperative recurrence rate is high. Mid-to-lower rectal cancer is close to the anal sphincter, making it difficult to preserve the anus and its function during surgery, which is a major challenge and a highly debated issue in surgical approaches. In China, the median age of onset for rectal cancer is around 45 years, with an increasing trend in incidence among young people.
bubble_chart Clinical Manifestations
The early clinical features of rectal cancer mainly include hematochezia and changes in bowel habits. When the cancer is confined to the rectal mucosa, hematochezia accounts for 85% of the sole early symptoms, but unfortunately, it is often overlooked by patients. At this stage, a digital rectal examination can often detect a mass. In patients with intermediate or advanced rectal cancer, in addition to common systemic symptoms such as loss of appetite, weight loss, and anemia, there are also local irritative symptoms caused by the tumor, including increased frequency of bowel movements, incomplete evacuation, frequent urge to defecate, and tenesmus. Enlargement of the tumor can lead to narrowing of the intestinal lumen, resulting in signs of intestinal obstruction. If the tumor invades surrounding tissues and organs, it may cause symptoms such as dysuria, frequent urination, and painful urination. Invasion of the presacral nerve plexus can lead to pain in the sacrococcygeal region and lower back. Metastasis to the liver can cause hepatomegaly, ascites, jaundice, and even cachexia.
bubble_chart DiagnosisIn clinical practice, patients with bloody stools should be treated with high vigilance. They should not be hastily diagnosed as "dysentery" or "internal hemorrhoids." Further examinations must be conducted to rule out the possibility of cancer. For the early diagnosis of {|###|}intestinal cancer{|###|}, the application of methods such as digital rectal examination, proctoscopy, or sigmoidoscopy must be emphasized.
(1) Digital Rectal Examination Approximately 90% of {|###|}intestinal cancers{|###|}, especially cancers in the lower rectum, can be detected by digital examination alone. However, some physicians still neglect this routine examination for patients suspected of having {|###|}intestinal cancer{|###|}, leading to delayed diagnosis and treatment. In fact, this diagnostic method is simple and feasible. Through digital rectal examination, the size and extent of infiltration of the palpable mass, whether it is fixed, and the presence of extramural or pelvic metastatic masses can also be assessed.
(2) Proctoscopy or Sigmoidoscopy After a digital rectal examination, proctoscopy should be performed to assist in diagnosis under direct vision, observing the morphology of the mass, its upper and lower edges, and its distance from the anal verge. Tissue from the mass should be taken for pathological biopsy to determine the nature of the mass and its degree of differentiation. For cancers located in the middle or upper rectum, which cannot be reached by finger examination, sigmoidoscopy is a preferable method.
(3) Barium Enema and Fiber Colonoscopy These methods are of limited help in diagnosing {|###|}intestinal cancer{|###|} and are therefore not considered routine examinations. They are only used to exclude the possibility of multiple colorectal tumors.
bubble_chart Treatment MeasuresThe treatment of rectal cancer is still primarily based on surgical intervention, supplemented by chemotherapy, with radiotherapy playing a certain role.
(1) Surgical treatment is divided into radical and palliative approaches.
1. Radical surgery: The surgical method is determined by the location of the tumor in the rectum. Within the rectal wall, there are two lymphatic systems: the submucosal lymphatic plexus and the intermuscular lymphatic plexus. Metastasis of cancer cells within the lymphatic system of the intestinal wall is relatively rare. Once cancer cells penetrate the intestinal wall, they spread to the extramural lymphatic system. Generally, they first involve the paracolic lymph nodes at the same level or slightly higher than the tumor, then progressively ascend to involve the intermediate lymph node groups accompanying the superior hemorrhoidal artery, and finally reach the lymph node groups near the inferior mesenteric artery (Figure 1). This upward lymphatic metastasis is the most common mode of spread in rectal cancer. If the tumor is located in the lower rectum, cancer cells may also spread laterally along the lymphatic vessels of the levator ani muscle and pelvic fascia to the obturator lymph nodes or follow the middle hemorrhoidal artery to the internal iliac lymph nodes. Occasionally, cancer cells may descend through the levator ani muscle, following the inferior hemorrhoidal artery to the lymph nodes in the ischiorectal fossa and inguinal lymph nodes. Since lymphatic metastasis in upper rectal cancer almost always ascends, surgical removal of the lymph nodes adjacent to the tumor and those above this level can achieve radical cure, with the possibility of preserving the anal sphincter. Although lymphatic metastasis in lower rectal cancer mainly ascends, there is also the possibility of lateral spread to the internal iliac and obturator lymph nodes. Radical surgery must include the perirectal tissues and levator ani muscle, making it impossible to preserve the anal sphincter. Specific surgical methods are as follows:
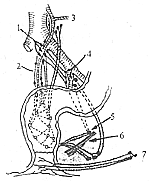
Figure 1: Drainage pathways of the intra- and extramural lymphatic systems of the rectum
The dotted network indicates the intramural lymphatic system
1. Sigmoid artery 2. Superior hemorrhoidal artery 3. Left colic artery 4. Internal iliac lymph nodes 5. Obturator lymph nodes 6. Levator ani muscle 7. Inguinal lymph nodes
(1) Abdominoperineal resection (Miles operation): Suitable for lower rectal cancer within 7 cm of the anal verge. The resection includes the sigmoid colon and its mesentery, rectum, anal canal, levator ani muscle, tissues in the ischiorectal fossa, and perianal skin. The vessels are ligated and divided at the root of the inferior mesenteric artery or below the origin of the left colic artery, with clearance of the corresponding lymph nodes. A permanent colostomy is created in the abdomen, and the perineal wound is either sutured primarily or packed with gauze. This procedure ensures thorough removal and a high cure rate (Figure 2).
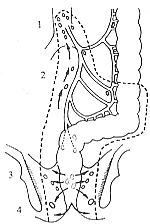
Figure 2: Abdominoperineal resection for rectal cancer
The dotted line indicates the resection range
1. Lymph nodes around the main artery 2. Lymph nodes of the sigmoid mesentery 3. Lymph nodes above the levator ani muscle 4. Lymph nodes in the ischioanal space
(2) Low anterior resection with extraperitoneal primary anastomosis, also known as anterior resection for rectal cancer (Dixon operation), is suitable for upper rectal cancer more than 12 cm from the anal verge. It involves intra-abdominal resection of the sigmoid colon and most of the rectum, mobilization of the rectum below the peritoneal reflection, and extraperitoneal anastomosis of the sigmoid colon and rectal stump. This procedure is less invasive and preserves the original anal sphincter, making it ideal. However, it is not suitable if the tumor is large and has infiltrated surrounding tissues.
(3) Sphincter-preserving rectal cancer resection: Suitable for early-stage rectal cancer located 7–11 cm from the anal verge. If the tumor is large, poorly differentiated, or if the main upward lymphatic vessels are obstructed by cancer cells with lateral lymphatic metastasis, this procedure may not achieve complete resection, and abdominoperineal resection is preferable. Currently used sphincter-preserving rectal cancer resection techniques include anastomosis using a stapler, low anterior resection with transanal eversion anastomosis, abdominal mobilization with transanal pull-through resection and anastomosis, and abdominosacral resection. The appropriate method can be selected based on the specific circumstances.
2. Palliative Surgery If the cancer has severely infiltrated locally or metastasized extensively and cannot be radically treated, palliative resection may be performed to relieve obstruction and reduce patient suffering. This involves limited removal of the cancerous intestinal segment, suturing the distal rectal stump, and creating a colostomy using the sigmoid colon (Hartmann's procedure). If this is not feasible, only a sigmoid colostomy may be performed, especially in patients already presenting with intestinal obstruction.
(II) Chemotherapy Same as for intestinal cancer.
(III) Radiotherapy The role of radiotherapy in the treatment of rectal intestinal cancer has gained increasing attention. It includes two approaches: combined therapy with surgery and radiotherapy alone.
1. Combined Surgery and Radiotherapy ① Preoperative radiotherapy can control the primary lesion and lymph node metastasis, improve resection rates, and reduce local recurrence. It is suitable for Stage III (Dukes C) rectal intestinal cancer. A pelvic anterior-posterior two-field opposing irradiation is used, with a radiation dose of 40–45 Gy (4000–4500 rad). Surgery is performed 3 weeks after radiotherapy. ② Postoperative radiotherapy is indicated for cases with pathologically confirmed lymph node metastasis, significant tumor infiltration beyond the intestinal wall, or unresectable residual lesions in the pelvis. It usually begins 1–2 months postoperatively, after the perineal wound has healed. Pelvic anterior-posterior two-field irradiation is used, sometimes supplemented with perineal field irradiation. The radiation dose can reach 45–50 Gy (4500–5000 rad).
(IV) Local Tumor Cryotherapy, Laser, and Cauterization For advanced-stage rectal intestinal cancer patients with signs of incomplete intestinal obstruction, local tumor cryotherapy or cauterization (including electrocautery and chemical cauterization) may be attempted to shrink or ablate the tumor tissue, temporarily relieving obstruction symptoms. In recent years, laser therapy has been introduced, using Nd-YAG laser at 65 W power for focal irradiation of the tumor tissue. If bleeding occurs, the power is reduced to 40 W for focused irradiation around the bleeding site to achieve hemostasis. Repeat irradiation every 2–3 weeks may lead to tumor shrinkage in some cases, temporarily alleviating symptoms. This can serve as a palliative treatment method.
(V) Treatment for Metastatic and Recurrent Patients
1. Treatment of Local Recurrence If the local recurrence is limited in scope and there are no other sites of recurrence or metastasis, surgical exploration may be attempted for resection. If the recurrence is confined to the center of the perineal incision and has not extended to the ischial tuberosities on either side, wide resection may be possible. However, if the perineal nodule or mass represents a pelvic recurrence extending to the perineal root, surgery is not advisable, as complete resection is unachievable and may instead result in incising the tumor tissue, leaving a non-healing wound.
For pelvic recurrent lesions, radiotherapy may be administered at 20 Gy (2000 rad) per course to temporarily alleviate pain symptoms.
2. Treatment of Liver Metastasis Recent studies have shown that surgical resection of metastatic lesions in rectal intestinal cancer is not as pessimistic as previously thought. If liver metastases can be resected along with the primary lesion, survival rates may improve. For solitary metastases, liver segmentectomy or wedge resection may be performed. For multiple unresectable liver metastases, de-arterialization measures may be employed first, such as ligating the hepatic artery to induce tumor necrosis, followed by catheter insertion into the distal hepatic artery for infusion of fluorouracil and mitomycin. Hepatic artery embolization may also be used to significantly reduce tumor volume. However, these treatments are contraindicated in patients with significant jaundice, severe liver dysfunction, portal vein obstruction, or those over 65 years old. Radiotherapy may improve symptoms in some patients. Recent reports have explored radiofrequency hyperthermia for liver metastases, with oral metronidazole potentially enhancing the anticancer effect. The efficacy is still under evaluation.
According to the survival rate analysis of 99,552 cases of malignant tumors in Shanghai from 1972 to 1979 by the Shanghai Cancer Institute, the 5-year survival rate of 6,905 cases of large intestine cancer (including rectal cancer) was 27.8%, significantly higher than that of malignant tumors of the stomach, lung, liver, esophagus, and pancreas. A follow-up study by the Cancer Hospital affiliated with Shanghai Medical University on 1,385 cases of surgically resected large intestine cancer showed that among them, the 5-year and 10-year survival rates of 1,061 cases of rectal cancer were 47.20% and 40.28%, respectively, slightly lower than the 54.63% and 53.90% for 324 cases of colon cancer. The median survival time for 118 patients who underwent palliative resection for rectal cancer was 15 months (range: 3–50 months), while the median survival time for 202 patients with unresectable rectal cancer was 8 months (range: 1–96 months). Similarly, the prognosis of rectal cancer was unrelated to the patient's gender or age but was closely associated with the disease course, extent of tumor infiltration, degree of differentiation, and presence or absence of metastasis.




